Preclinical validation of human recombinant glutamate-oxaloacetate transaminase for the treatment of acute ischemic stroke
- PMID: 39524351
- PMCID: PMC11543921
- DOI: 10.1016/j.isci.2024.111108
Preclinical validation of human recombinant glutamate-oxaloacetate transaminase for the treatment of acute ischemic stroke
Abstract
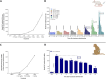
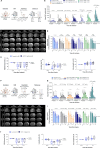
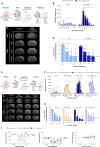
The blood enzyme glutamate-oxaloacetate transaminase (GOT) has been postulated as an effective therapeutic to protect the brain during stroke. To demonstrate its potential clinical utility, a new human recombinant form of GOT (rGOT) was produced for medical use. We tested the pharmacokinetics and evaluated the protective efficacy of rGOT in rodent and non-human primate models that reflected clinical stroke conditions. We found that continuous intravenous administration of rGOT within the first 8 h after ischemic onset significantly reduced the infarct size in both severe (30%) and mild lesions (48%). Cerebrospinal fluid and proteomics analysis, in combination with positron emission tomography imaging, indicated that rGOT can reach the brain and induce cytoprotective autophagy and induce local protection by alleviating neuronal apoptosis. Our results suggest that rGOT can be safely used immediately in patients suspected of having a stroke. This study requires further validation in clinical stroke populations.
Keywords: biological sciences; natural sciences; neuroscience; pharmacology; physiology.
© 2024 The Author(s).
Conflict of interest statement
The funding sponsors did not participate in the study design; collection, analysis, or interpretation of data; writing the report; or in the decision to submit the paper for publication. Preparation of the new version of identical human rGOT at Biotechpharma (Lithuania) was supported by a grant from Sun Pharma (Mumbai, India) to Prof. David Mirelman.
Figures





References
-
- Rej R. Measurement of aspartate aminotransferase activity: effects of oxamate. Clin. Chem. 1979;25:555–559. - PubMed
-
- Rej R. Aspartate aminotransferase activity and isoenzyme proportions in human liver tissues. Clin. Chem. 1978;24:1971–1979. - PubMed
-
- Abrego J., Gunda V., Vernucci E., Shukla S.K., King R.J., Dasgupta A., Goode G., Murthy D., Yu F., Singh P.K. GOT1-mediated anaplerotic glutamine metabolism regulates chronic acidosis stress in pancreatic cancer cells. Cancer Lett. 2017;400:37–46. doi: 10.1016/j.canlet.2017.04.029. - DOI - PMC - PubMed
-
- Vijay Kumar K.D.G. Springer; 2018. To Determine Alanine and Aspartate Transaminase Activity in Serum. - DOI
-
- Zhang D., Xiao M., Wang L., Jia W. Blood-Based Glutamate Scavengers Reverse Traumatic Brain Injury-Induced Synaptic Plasticity Disruption by Decreasing Glutamate Level in Hippocampus Interstitial Fluid, but Not Cerebral Spinal Fluid. Neurotox. Res. 2019;35:360–372. doi: 10.1007/s12640-018-9961-8. - DOI - PubMed
LinkOut - more resources
Full Text Sources

