Intra-tumoral YAP and TAZ heterogeneity drives collective NSCLC invasion that is targeted by SUMOylation inhibitor TAK-981
- PMID: 39524367
- PMCID: PMC11544388
- DOI: 10.1016/j.isci.2024.111133
Intra-tumoral YAP and TAZ heterogeneity drives collective NSCLC invasion that is targeted by SUMOylation inhibitor TAK-981
Abstract
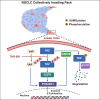
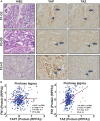
Non-small cell lung cancer (NSCLC) collective invasion is supported by cooperativity of proliferative (follower) and invasive (leader) cells. H1299-isolated follower cells exhibit higher Yes-associated protein (YAP) expression, while leader cells were found to express elevated transcriptional coactivator with PDZ-binding motif (TAZ/WWTR1) expression. Suppressing TAZ (not YAP) in leader cells reduced invasion. TAZ-regulated leader cell invasion is associated with activation of the EGFR-PI3K-AKT axis. NSCLC patient samples also demonstrated heterogeneity in YAP and TAZ expression. YAP and TAZ regulate proliferation of follower and leader cells. Our results highlight the need to inhibit both YAP and TAZ to effectively target their regulation of collective invasion. We identify that the SUMOylation inhibitor TAK-981 reduces YAP and TAZ expression, decreasing tumor burden and metastasis in a murine NSCLC model. Our study reveals an intra-tumoral division of labor, driven by differential YAP and TAZ expression, which can be effectively targeted with TAK-981 for NSCLC therapy.
Keywords: Cancer; Cell biology; Microenvironment.
© 2024 The Author(s).
Conflict of interest statement
T.L. is on the advisory board for Mirati, AstraZeneca, Merck, Takeda, EMD Serono, Eisai, and Jazz; is a consultant for Merck, Daiichi-Sankyo, Janssen, Eisai, Novocure, Amgen, Roche, Regeneron, and Catalyst; and has research funding to institution from Pfizer, Advaxis, and Bayer.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

