Quantification of autoantibodies using a luminescent profiling method in autoimmune interstitial lung disease
- PMID: 39524452
- PMCID: PMC11543486
- DOI: 10.3389/fimmu.2024.1462242
Quantification of autoantibodies using a luminescent profiling method in autoimmune interstitial lung disease
Abstract
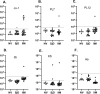
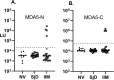
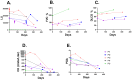
Autoantibodies are important for the diagnosis of autoimmune interstitial lung disease (ILD). Standard immunoassays have limitations, including their qualitative nature and/or a narrow dynamic range of detection, hindering the usefulness of autoantibodies as biomarkers of disease activity. Here, the luciferase immunoprecipitation system (LIPS) was evaluated for measuring myositis-specific and other lung-related autoantibodies in 25 subjects with idiopathic inflammatory myopathies (IIM), 26 with Sjögren's disease (SjD), and 10 healthy volunteers. LIPS detected a broad dynamic range of autoantibodies, to MDA5, Jo-1, PL12, KS, U1-70K, and Ro52, and matched seropositivity status with established immunoassays. Robust anti-MDA5 autoantibodies in four IIM-ILD patients had a median value of 1,134,000 LU (IQR 473,000-2,317,000), which was 500 times higher than in 21 seronegative IIM patients. Markedly elevated anti-Jo-1 autoantibodies in five IIM-ILD patients demonstrated a median value of 1,177,000 LU (IQR: 604,000-2,520,000), which was 1000-fold higher than in seronegative patients. Robust anti-Ro52 and other anti-tRNA-synthetase autoantibodies were detected in a subset of IIM-ILD subjects. In SjD, only anti-U1-70K and KS autoantibodies were identified in ILD patients with a prevalence of 30% and 20%, respectively. In longitudinal samples of five IIM-ILD patients, anti-Jo-1 autoantibody levels paralleled clinical improvement of lung function. LIPS can accurately quantify autoantibody levels as biomarkers for treatment response in patients with autoimmune ILD.
Keywords: Sjögren’s disease; idiopathic inflammatory myopathies; interstitial lung disease; myositis-associated autoantibody; myositis-specific autoantibody.
Copyright © 2024 Burbelo, Huapaya, Khavandgar, Beach, Pinal-Fernandez, Mammen, Chiorini, Noroozi Farhadi, Miller, Schiffenbauer, Sarkar, Warner and Rider.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
-
- Sato S, Hoshino K, Satoh T, Fujita T, Kawakami Y, Fujita T, et al. . RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: Association with rapidly progressive interstitial lung disease. Arthritis Rheumatol. (2009) 60:2193–200. doi: 10.1002/art.24621 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

