Successful Targeting of Somatic VHL Alterations With Belzutifan in Two Cases
- PMID: 39524464
- PMCID: PMC11541926
- DOI: 10.36401/JIPO-24-13
Successful Targeting of Somatic VHL Alterations With Belzutifan in Two Cases
Abstract
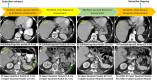
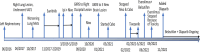
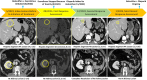
Clear cell renal cell carcinoma (RCC) is commonly associated with alterations in the VHL tumor suppressor gene, resulting in upregulation of hypoxia-inducible factor pathways. Immune checkpoint inhibitors and vascular endothelial growth factor inhibitors are the mainstays of systemic treatment for metastatic RCC; however, most patients encounter disease progression after the initial response. The phase 3 clinical trial LITESPARK-005-belzutifan (HIF-2α inhibitor) demonstrated improvement in progression-free survival compared with everolimus in heavily pretreated patients unselected for somatic/germline VHL alterations (an objective response rate of 23% and a median time on therapy of 7.6 months in the belzutifan cohort), resulting in U.S. FDA approval for patients with advanced RCC. Herein, we present two cases of refractory metastatic RCC (including one with brain metastases) with somatic VHL mutations who received belzutifan after discussion in the institutional Molecular Tumor Board. Both patients had an excellent clinical response (partial remissions ongoing at >12 and >20 months). Future studies should assess the merits of biomarker selection for belzutifan treatment.
Keywords: HIF; HIF-2α; VHL alterations; belzutifan; renal cell carcinoma.
Conflict of interest statement
Conflicts of Interest: Aditya Shreenivas serves on the advisory board of Taiho Oncology and Iylon Pvt. Ltd. and reports research funding from Natera and Caris. Hui-Zi Chen serves on the advisory board of Jazz Pharmaceuticals and reports speaker fees from Jazz Pharmaceuticals. Ben George reports research funding (to the institution) from Roche/Genentech, Hoffman La-Roche, Taiho Oncology, Boehringer Ingelheim, Toray, NGM Biopharma, Hutchison Medipharma, Mirati Therapeutics, CARsgen, Glyconex, Helix Biopharma, Pfizer, Transcenta, and TVARDI; and consultant/speaker fees from Ipsen, Bristol Myers Squibb, Foundation Medicine, Taiho Oncology, BTG (Boston Scientific), Roche/Genentech, France Foundation, and AztraZeneca. Razelle Kurzrock reportsresearch funding from Boehringer Ingelheim, Debiopharm, Foundation Medicine, Genentech, Grifols, Guardant, Incyte, Konica Minolta, Medimmune, Merck Serono, Omniseq, Pfizer, Sequenom, Takeda, TopAlliance, and from the NCI; consultant and/or speaker fees and/or advisory board/consultant for Actuate Therapeutics, AstraZeneca, Bicara Therapeutics, Inc., Biological Dynamics, Caris, Datar Cancer Genetics, Daiichi, EISAI, EOM Pharmaceuticals, Iylon, LabCorp, Merck, NeoGenomics, Neomed, Pfizer, Precirix, Prosperdtx, Regeneron, Roche, TD2/Volastra, Turning Point Therapeutics, and X-Biotech; equity interest in CureMatch Inc. and IDbyDNA; board member of CureMatch and CureMetrix; co-founder of CureMatch. The remaining authors have no disclosures.
Figures

References
-
- Motzer RJ, Escudier B, McDermott DF, et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J Immunother Cancer. 2020;8:e000891. - PMC - PubMed
-
- Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–1127. - PubMed
-
- Choueiri TK, Powles T, Burotto M, et al. 696O_PR Nivolumab + cabozantinib vs sunitinib in first-line treatment for advanced renal cell carcinoma: First results from the randomized phase III CheckMate 9ER trial. Ann Oncol. 2020;31:S1159.
Publication types
LinkOut - more resources
Full Text Sources
