Targeting the Plasmodium falciparum IspE Enzyme
- PMID: 39524657
- PMCID: PMC11541488
- DOI: 10.1021/acsomega.4c06038
Targeting the Plasmodium falciparum IspE Enzyme
Abstract
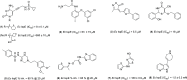
The enzyme IspE in Plasmodium falciparum is considered an attractive drug target, as it is essential for parasite survival and is absent in the human proteome. Yet it still has not been addressed by a small-molecule inhibitor. In this study, we conducted a high-throughput screening campaign against the PfIspE enzyme. Our approach toward a PfIspE inhibitor comprises in vitro screening, structure-activity relationship studies, examining the docking position using an AlphaFold model, and finally target verification through probe binding and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. The newly synthesized probe containing a diazirine and an alkyne moiety (23) allowed us to demonstrate its binding to IspE in the presence of a lysate of human cells (HEK293 cells) and to get evidence that both probe 23 and the best inhibitor of the series (19) compete for the same IspE binding site.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
LinkOut - more resources
Full Text Sources
