Sleeve-gastrectomy results in improved metabolism and a massive stress response of the liver proteome in a mouse model of metabolic dysfunction-associated steatohepatitis
- PMID: 39524892
- PMCID: PMC11550656
- DOI: 10.1016/j.heliyon.2024.e38678
Sleeve-gastrectomy results in improved metabolism and a massive stress response of the liver proteome in a mouse model of metabolic dysfunction-associated steatohepatitis
Abstract
Background: Bariatric surgery has been shown to improve the histopathological findings in patients with obesity and metabolic dysfunction-associated steatohepatitis, but there are also reports about non-responders or progressive disease after bariatric interventions. Therefore, it is of utmost importance to understand the pathophysiological processes in the liver after bariatric surgery.
Materials and methods: In the present study, 4 weeks old male C57/Bl6 mice were fed a Western Diet to induce metabolic dysfunction-associated steatohepatitis and sleeve-gastrectomy (SG), or sham operation in the pair-fed and ad libitum control group were performed. Mice were observed for two or eight weeks after surgery and metabolic assessment was performed throughout the experiment. Histopathology, flow cytometry and proteomic analyses were conducted to evaluate hepatic inflammation, liver metabolism and affected signaling pathways.
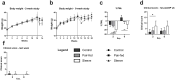
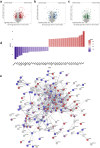
Results: Weight loss was higher, and metabolism significantly improved after SG. Two weeks after SG major inflammatory and regulatory disturbances in the liver were observed. The proportion of hepatic CD3+NK1.1+ cells were decreased, and proteins involved in apoptosis like Fas, Casp1 and Casp9 or in the acute phase response were upregulated in SG mice. These disturbances decreased in the long-term and we observed an increase of many proteins involved in lipid metabolism eight weeks following SG.
Conclusions: The rapid weight loss and decrease of hepatic fat after SG lead to a proinflammatory response in the liver in the early phase after surgery, which changes to a more moderate immune response in the long-term. We suggest a preoperative risk stratification and postoperative surveillance depending on the histopathological findings.
Keywords: Bariatric surgery; Metabolic dysfunction-associated steatohepatitis; Metabolic dysfunction-associated steatotic liver disease; Nonalcoholic fatty liver diseases; Nonalcoholic steatohepatitis; Sleeve-gastrectomy.
© 2024 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Andreas Kroh reports financial support was provided by B. Braun Foundation, Melsungen, Germany. All other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Multiomics analyses decipher intricate changes in the cellular and metabolic landscape of steatotic livers upon dietary restriction and sleeve gastrectomy.Int J Biol Sci. 2024 Aug 19;20(11):4438-4457. doi: 10.7150/ijbs.98362. eCollection 2024. Int J Biol Sci. 2024. PMID: 39247824 Free PMC article.
-
Lipid metabolic reprogramming mediated by circulating Nrg4 alleviates metabolic dysfunction-associated steatotic liver disease during the early recovery phase after sleeve gastrectomy.BMC Med. 2024 Apr 17;22(1):164. doi: 10.1186/s12916-024-03377-0. BMC Med. 2024. PMID: 38632600 Free PMC article.
-
Effects of sleeve gastrectomy in high fat diet-induced obese mice: respective role of reduced caloric intake, white adipose tissue inflammation and changes in adipose tissue and ectopic fat depots.Surg Endosc. 2014 Feb;28(2):592-602. doi: 10.1007/s00464-013-3211-1. Epub 2013 Oct 3. Surg Endosc. 2014. PMID: 24196540
-
Sleeve gastrectomy ameliorated high-fat diet (HFD)-induced non-alcoholic fatty liver disease and upregulated the nicotinamide adenine dinucleotide +/ Sirtuin-1 pathway in mice.Asian J Surg. 2021 Jan;44(1):213-220. doi: 10.1016/j.asjsur.2020.05.030. Epub 2020 Jul 22. Asian J Surg. 2021. PMID: 32712045
-
Early Weight Loss Independent Effects of Sleeve Gastrectomy on Diet-Induced Cardiac Dysfunction in Obese, Wistar Rats.Obes Surg. 2017 Sep;27(9):2370-2377. doi: 10.1007/s11695-017-2632-7. Obes Surg. 2017. PMID: 28299572 Free PMC article.
References
-
- Williams C.D., Stengel J., Asike M.I., Torres D.M., Shaw J., Contreras M., et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140(1):124–131. - PubMed
-
- Wolk A., Gridley G., Svensson M., Nyren O., McLaughlin J.K., Fraumeni J.F., Adam H.O. A prospective study of obesity and cancer risk (Sweden) Cancer Causes Control. 2001;12(1):13–21. - PubMed
-
- Calle E.E., Rodriguez C., Walker-Thurmond K., Thun M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003;348(17):1625–1638. - PubMed
-
- Moller H., Mellemgaard A., Lindvig K., Olsen J.H. Obesity and cancer risk: a Danish record-linkage study. Eur. J. Cancer. 1994;30A(3):344–350. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

