Dexmedetomidine preconditioning attenuates ferroptosis in myocardial ischemia-reperfusion injury via α2 adrenergic receptor activation
- PMID: 39524900
- PMCID: PMC11544042
- DOI: 10.1016/j.heliyon.2024.e39697
Dexmedetomidine preconditioning attenuates ferroptosis in myocardial ischemia-reperfusion injury via α2 adrenergic receptor activation
Abstract
Objective: Dexmedetomidine (Dex) is a potent agonist of the α2 adrenergic receptor that has been shown to possess sedative and hypnotic properties. Dex can protect against myocardial ischemia-reperfusion injury (MIRI) by inhibiting ferroptosis. However, these studies were based on Dex post-conditioning, and the role of α2 adrenergic receptors in this process is unclear. In this study, we investigated whether Dex preconditioning can prevent MIRI by attenuating ferroptosis and whether this effect depends on α2 adrenergic receptors in rats.
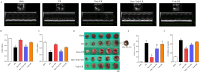
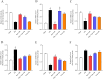
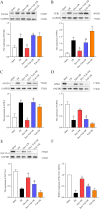
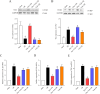
Methods: Male Sprague-Dawley rats were randomly assigned to five groups: sham (saline-treated), I/R (ischemia-exposed), Dex + I/R (Dex pre-treatment), Dex + Yoh + I/R (Dex and yohimbine pre-treatment), and Yoh + I/R (yohimbine pre-treatment). Cardiac function, myocardial infarction, and morphological changes were assessed. Transmission electron microscopy was used to analyze mitochondrial morphology. Ferroptosis-related indicators and lipid peroxidation were measured using western blotting and qRT-PCR.
Results: Our findings indicated that Dex preconditioning improved cardiac function, reduced infarct size and apoptosis, and inhibited ferroptosis in the rat myocardium after MIRI. These effects were associated with the upregulation of Nrf2, SLC7A11, and GPX4 expression, as well as the downregulation of Ferritin, TFR1, ACSL4, COX2, IL-1β, IL-6, and TNF-α expression. Importantly, yohimbine, an α2 adrenergic receptor antagonist, abolished these protective effects.
Conclusion: These results suggest that Dex preconditioning can prevent MIRI by attenuating ferroptosis via α2 adrenergic receptor activation and by modulating the Nrf2/SLC7A11/GPX4 pathway.
Keywords: Dexmedetomidine; Ferroptosis; Myocardial ischemia-reperfusion injury; SLC7A11; α2 adrenergic receptor.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Dexmedetomidine Preconditioning Attenuates Myocardial Ischemia/Reperfusion Injury in Rats by Suppressing Mitophagy Via Activating Α2-Adrenergic Receptor.Arq Bras Cardiol. 2023 Oct;120(10):e20220750. doi: 10.36660/abc.20220750. Arq Bras Cardiol. 2023. PMID: 37909577 Free PMC article. English, Portuguese.
-
Dexmedetomidine preconditioning may attenuate myocardial ischemia/reperfusion injury by down-regulating the HMGB1-TLR4-MyD88-NF-кB signaling pathway.PLoS One. 2017 Feb 21;12(2):e0172006. doi: 10.1371/journal.pone.0172006. eCollection 2017. PLoS One. 2017. PMID: 28222157 Free PMC article.
-
Dexmedetomidine Attenuates Ferroptosis-Mediated Renal Ischemia/Reperfusion Injury and Inflammation by Inhibiting ACSL4 via α2-AR.Front Pharmacol. 2022 Jun 14;13:782466. doi: 10.3389/fphar.2022.782466. eCollection 2022. Front Pharmacol. 2022. PMID: 35873574 Free PMC article.
-
Dexmedetomidine attenuates myocardial ischemia/reperfusion-induced ferroptosis via AMPK/GSK-3β/Nrf2 axis.Biomed Pharmacother. 2022 Oct;154:113572. doi: 10.1016/j.biopha.2022.113572. Epub 2022 Aug 18. Biomed Pharmacother. 2022. PMID: 35988428
-
Dexmedetomidine post-conditioning alleviates myocardial ischemia-reperfusion injury in rats by ferroptosis inhibition via SLC7A11/GPX4 axis activation.Hum Cell. 2022 May;35(3):836-848. doi: 10.1007/s13577-022-00682-9. Epub 2022 Feb 25. Hum Cell. 2022. PMID: 35212945
Cited by
-
The interaction between ferroptosis and myocardial ischemia-reperfusion injury: molecular mechanisms and potential therapeutic targets.Eur J Med Res. 2025 Jul 21;30(1):643. doi: 10.1186/s40001-025-02851-6. Eur J Med Res. 2025. PMID: 40685346 Free PMC article. Review.
References
-
- Ottani F., Limbruno U., Latini R., et al. Reperfusion in STEMI patients: still a role for cardioprotection? Minerva Cardioangiol. 2018;66(4):452–463. - PubMed
-
- Xiang Q., Yi X., Zhu X.H., Wei X., Jiang D.S. Regulated cell death in myocardial ischemia-reperfusion injury. Trends Endocrinol. Metabol. 2023;S1043–2760(23) 00223-0 [pii] - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

