Temperature and Ultrasound-Responsive Nanoassemblies for Enhanced Organ Targeting and Reduced Cardiac Toxicity
- PMID: 39524922
- PMCID: PMC11550713
- DOI: 10.2147/IJN.S470465
Temperature and Ultrasound-Responsive Nanoassemblies for Enhanced Organ Targeting and Reduced Cardiac Toxicity
Abstract
Background: Biocompatible nanocarriers are widely employed as drug-delivery vehicles for treatment. Nevertheless, indiscriminate drug release, insufficient organ-specific targeting, and systemic toxicity hamper nanocarrier effectiveness. Stimuli-responsive nano-sized drug delivery systems (DDS) are an important strategy for enhancing drug delivery efficiency and reducing unexpected drug release.
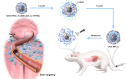
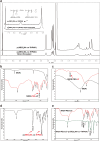
Methods: This study introduces a temperature- and ultrasound-responsive nano-DDS in which the copolymer p-(MEO2MA-co-THPMA) is grafted onto mesoporous iron oxide nanoparticles (MIONs) to construct an MPL-p nano-DDS. The copolymer acts as a nanopore gatekeeper, assuming an open conformation at sub-physiological temperatures that allows drug encapsulation and a closed conformation at physiological temperatures that prevents unexpected drug release during circulation. Lactoferrin was conjugated to the nanoparticle surface via polyethylene glycol to gain organ-targeting ability. External ultrasonic irradiation of the nanoparticles in the targeted organs caused a conformational change of the copolymer and reopened the pores, facilitating controlled drug release.
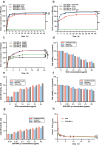
Results: MPL-p exhibited excellent biocompatibility and rare drug release in circulation. When targeting delivery to the brain, ultrasound promoted the release of the loaded drugs in the brain without accumulation in other organs, avoiding the related adverse reactions, specifically those affecting the heart.
Conclusion: This study established a novel temperature- and ultrasound-responsive DDS that reduced systemic adverse reactions compared with traditional DDS, especially in the heart, and demonstrated excellent organ delivery efficiency.
Keywords: drug delivery; mesoporous iron oxide nanoparticles; systemic toxicity; temperature- and ultrasound-responsive.
© 2024 Jiang et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures





Similar articles
-
Thermoresponsive polymer gated and superparamagnetic nanoparticle embedded hollow mesoporous silica nanoparticles as smart multifunctional nanocarrier for targeted and controlled delivery of doxorubicin.Nanotechnology. 2020 Nov 6;31(45):455604. doi: 10.1088/1361-6528/ab8b0e. Epub 2020 Apr 20. Nanotechnology. 2020. PMID: 32311684
-
Release of a liver anticancer drug, sorafenib from its PVA/LDH- and PEG/LDH-coated iron oxide nanoparticles for drug delivery applications.Sci Rep. 2020 Dec 9;10(1):21521. doi: 10.1038/s41598-020-76504-5. Sci Rep. 2020. PMID: 33298980 Free PMC article.
-
Hierarchically Self-Assembled Supramolecular Host-Guest Delivery System for Drug Resistant Cancer Therapy.Biomacromolecules. 2018 Jun 11;19(6):1926-1938. doi: 10.1021/acs.biomac.7b01693. Epub 2018 Feb 1. Biomacromolecules. 2018. PMID: 29350902
-
Emerging Strategies in Stimuli-Responsive Nanocarriers as the Drug Delivery System for Enhanced Cancer Therapy.Curr Pharm Des. 2019;25(24):2609-2625. doi: 10.2174/1381612825666190709221141. Curr Pharm Des. 2019. PMID: 31603055 Review.
-
Endogenous and Exogenous Stimuli-Responsive Drug Delivery Systems for Programmed Site-Specific Release.Molecules. 2019 Mar 21;24(6):1117. doi: 10.3390/molecules24061117. Molecules. 2019. PMID: 30901827 Free PMC article. Review.
Cited by
-
Recent advances in nanoultrasonography for the diagnosis and treatment of gastrointestinal diseases.Nanomedicine (Lond). 2025 Mar;20(5):519-530. doi: 10.1080/17435889.2025.2457319. Epub 2025 Jan 23. Nanomedicine (Lond). 2025. PMID: 39846205 Review.
References
-
- Greish K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol Biol. 2010;624:25–37. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

