Exosomes Derived from Antler Mesenchymal Stem Cells Promote Wound Healing by miR-21-5p/STAT3 Axis
- PMID: 39524924
- PMCID: PMC11546281
- DOI: 10.2147/IJN.S481044
Exosomes Derived from Antler Mesenchymal Stem Cells Promote Wound Healing by miR-21-5p/STAT3 Axis
Abstract
Background: Deer antlers, unique among mammalian organs for their ability to regenerate annually without scar formation, provide an innovative model for regenerative medicine. This study explored the potential of exosomes derived from antler mesenchymal stem cells (AMSC-Exo) to enhance skin wound healing.
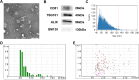
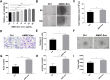
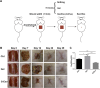
Methods: We explored the proliferation, migration and angiogenesis effects of AMSC-Exo on HaCaT cells and HUVEC cells. To investigate the skin repairing effect of AMSC-Exo, we established a full-thickness skin injury mouse model. Then the skin thickness, the epidermis, collagen fibers, CD31 and collagen expressions were tested by H&E staining, Masson's trichrome staining and immunofluorescence experiments. MiRNA omics analysis was conducted to explore the mechanism of AMSC-Exo in skin repairing.
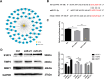
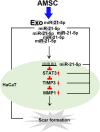
Results: AMSC-Exo stimulated the proliferation and migration of HaCaT cells, accelerated the migration and angiogenesis of HUVEC cells. In the mouse skin injury model, AMSC-Exo stimulated angiogenesis and regulated the extracellular matrix by facilitating the conversion of collagen type III to collagen type I, restoring epidermal thickness to normal state without aberrant hyperplasia. Notably, AMSC-Exo enhanced the quality of wound healing with increased vascularization and reduced scar formation. MiRNAs in AMSC-Exo, especially through the miR-21-5p/STAT3 signaling pathway, played a crucial role in these processes.
Conclusion: This study underscores the efficacy of AMSC-Exo in treating skin wounds, suggesting a new approach for enhancing skin repair and regeneration.
Keywords: deer antler; exosomes; mesenchymal stem cells; microRNA; skin wound healing.
© 2024 Meng et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

