Photo-responsive decellularized small intestine submucosa hydrogels
- PMID: 39525288
- PMCID: PMC11546089
- DOI: 10.1002/adfm.202401952
Photo-responsive decellularized small intestine submucosa hydrogels
Abstract
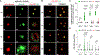
Decellularized small intestine submucosa (dSIS) is a promising biomaterial for promoting tissue regeneration. Isolated from the submucosal layer of animal jejunum, SIS is rich in extracellular matrix (ECM) proteins, including collagen, laminin, and fibronectin. Following mild decellularization, dSIS becomes an acellular matrix that supports cell adhesion, proliferation, and differentiation. Conventional dSIS matrix is usually obtained by thermal crosslinking, which yields a soft scaffold with low stability. To address these challenges, dSIS has been modified with methacrylate groups for photocrosslinking into stable hydrogels. However, dSIS has not been modified with clickable handles for orthogonal crosslinking. Here, we report the development of norbornene-modified dSIS, named dSIS-NB, via reacting amine groups of dSIS with carbic anhydride in acidic aqueous reaction conditions. Using triethylamine (TEA) as a mild base catalyst, we obtained high degrees of NB substitution on dSIS. In addition to describing the synthesis of dSIS-NB, we explored its adaptability in orthogonal hydrogel crosslinking and used dSIS-NB hydrogels for cancer and vascular tissue engineering. Impressively, compared with physically crosslinked dSIS and collagen matrices, orthogonally crosslinked dSIS-NB hydrogels supported rapid dissemination of cancer cells and superior vasculogenic and angiogenic properties. dSIS-NB was also exploited as a versatile bioink for 3D bioprinting applications.
Keywords: 3D bioprinting; Decellularized extracellular matrix; cancer engineering; functional vascularization; small intestine submucosa; thiol-norbornene.
Conflict of interest statement
Conflict of interest statement The authors have no conflicts of interest to declare.
Figures
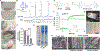



References
-
- Elomaa L, Gerbeth L, Almalla A, Fribiczer N, Daneshgar A, Tang P, Hillebrandt K, Seiffert S, Sauer IM, Siegmund B, Weinhart M, Additive Manufacturing 2023, 64, 103439;
- Palmosi T, Tolomeo AM, Cirillo C, Sandrin D, Sciro M, Negrisolo S, Todesco M, Caicci F, Santoro M, Dal Lago E, Marchesan M, Modesti M, Bagno A, Romanato F, Grumati P, Fabozzo A, Gerosa G, Frontiers in Bioengineering and Biotechnology 2022, 10. - PMC - PubMed
-
- U. S. F. a. D. Administration, 510(k) Premarket Notification, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K993948, accessed: August 25, 2023.
Grants and funding
LinkOut - more resources
Full Text Sources
