ZLN005 Reduces Neuroinflammation and Improves Mitochondrial Function in Mice with Perioperative Neurocognitive Disorders
- PMID: 39525311
- PMCID: PMC11545616
- DOI: 10.2147/JIR.S482051
ZLN005 Reduces Neuroinflammation and Improves Mitochondrial Function in Mice with Perioperative Neurocognitive Disorders
Abstract
Background: The decrease expression of PGC-1α contributes to perioperative neurocognitive disorders (PND). This study aimed to investigate the effects of the PGC-1α agonist ZLN005 in preventing PND and to explore the potential mechanism.
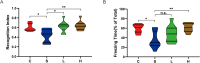
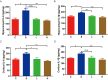
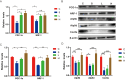
Methods: C57BL/6 mice were randomly divided into four groups: the control group (Group C), the surgery group (Group S), the surgery and ZLN005 (5 mg/(kg⋅d)) group (Group L), and the surgery and ZLN005 (7.5 mg/(kg⋅d)) group (Group H). Except for Group C, the other three groups received intraperitoneal injections of vehicle or ZLN005 once a day from 3 days before surgery to 3 days after surgery. The open field test, novel object recognition test and fear conditioning test were performed to measure anxiety behaviors, locomotor activity and memory. The levels of IL-6 and IL-1β were measured at 24 hours after surgery. ATP and ROS levels were measured at 3 days post-surgery. PGC-1α, NRF-1, Atp5d, Atp5k and Cox5a were measured at one day or three days post-surgery.
Results: ZLN005 treatment improved the cognitive function of mice in Group L and Group H compared with Group S. The expression of IL-6 and IL-1β in the hippocampus of the S group was increased after surgery, and ZLN005 reduced the expression of IL-6 and IL-1β in the hippocampus of mice one day after surgery. There were parallel decreases in the expression of PGC-1α/NRF-1 and mitochondrial function in the hippocampus of the Group S mice compared with the Group C mice. The expression of PGC-1α/NRF-1 and mitochondrial function were upregulated after ZLN005 treatment.
Conclusion: Neuroinflammation and mitochondrial damage are involved in the occurrence of PND. ZLN005 activates PGC-1α to increase the expression of mitochondrial proteins, improve mitochondrial function, and ultimately ameliorate the cognitive status of mice after surgery.
Keywords: PGC-1α; ZLN005; mitochondrial respiratory chain complex; neuroinflammation; perioperative neurocognitive disorders; respiratory function.
© 2024 Wu et al.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The PGC-1α/ERRα/ULK1 pathway contributes to Perioperative neurocognitive disorders by inducing mitochondrial dysfunction and activating NLRP3 inflammasome in aged mice.Neuropharmacology. 2024 Dec 1;260:110119. doi: 10.1016/j.neuropharm.2024.110119. Epub 2024 Aug 27. Neuropharmacology. 2024. PMID: 39197819
-
ZLN005 improves the protective effect of mitochondrial function on alveolar epithelial cell aging by upregulating PGC-1α.J Thorac Dis. 2023 Nov 30;15(11):6160-6177. doi: 10.21037/jtd-23-815. Epub 2023 Nov 27. J Thorac Dis. 2023. PMID: 38090292 Free PMC article.
-
Hippocampus-Based Mitochondrial Respiratory Function Decline Is Responsible for Perioperative Neurocognitive Disorders.Front Aging Neurosci. 2022 Feb 9;14:772066. doi: 10.3389/fnagi.2022.772066. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35221986 Free PMC article.
-
ZLN005 Alleviates In Vivo and In Vitro Renal Fibrosis via PGC-1α-Mediated Mitochondrial Homeostasis.Pharmaceuticals (Basel). 2022 Mar 31;15(4):434. doi: 10.3390/ph15040434. Pharmaceuticals (Basel). 2022. PMID: 35455432 Free PMC article.
-
Exploring the effect of Anshen Dingzhi prescription on hippocampal mitochondrial signals in single prolonged stress mouse model.J Ethnopharmacol. 2024 Apr 6;323:117713. doi: 10.1016/j.jep.2024.117713. Epub 2024 Jan 3. J Ethnopharmacol. 2024. PMID: 38181935
Cited by
-
Environmental enrichment attenuates sevoflurane anesthesia-induced learning deficits in aged mice through regulating TTBK1 and phosphorylated Tau expression.Exp Brain Res. 2025 Feb 14;243(3):67. doi: 10.1007/s00221-025-07017-8. Exp Brain Res. 2025. PMID: 39953251
References
LinkOut - more resources
Full Text Sources

