Progress and challenges in applying CRISPR/Cas techniques to the genome editing of trees
- PMID: 39525414
- PMCID: PMC11524270
- DOI: 10.48130/FR-2022-0006
Progress and challenges in applying CRISPR/Cas techniques to the genome editing of trees
Abstract
With the advent of the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/CRISPR-associated protein (Cas) system, plant genome editing has entered a new era of robust and precise editing for any genes of interest. The development of various CRISPR/Cas toolkits has enabled new genome editing outcomes that not only target indel mutations but also enable base editing and prime editing. The application of the CRISPR/Cas toolkits has rapidly advanced breeding and crop improvement of economically important species. CRISPR/Cas toolkits have also been applied to a wide variety of tree species, including apple, bamboo, Cannabaceae, cassava, citrus, cacao tree, coffee tree, grapevine, kiwifruit, pear, pomegranate, poplar, ratanjoyt, and rubber tree. The application of editing to these species has resulted in significant discoveries related to critical genes associated with growth, secondary metabolism, and stress and disease resistance. However, most studies on tree species have involved only preliminary optimization of editing techniques, and a more in-depth study of editing techniques for CRISPR/Cas-based editing of tree species has the potential to rapidly accelerate tree breeding and trait improvements. Moreover, tree genome editing still relies mostly on Cas9-based indel mutation and Agrobacterium-mediated stable transformation. Transient transformation for transgene-free genome editing is preferred, but it typically has very low efficiency in tree species, substantially limiting its potential utility. In this work, we summarize the current status of tree genome editing practices using the CRISPR/Cas system and discuss limitations that impede the efficient application of CRISPR/Cas toolkits for tree genome editing, as well as future prospects.
Keywords: Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated protein; Forest tree; Gene editing; Genetic transformation; Ribonucleo-protein complex.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
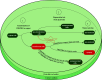
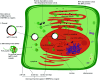
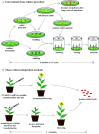
Similar articles
-
CRISPR/Cas: A powerful tool for gene function study and crop improvement.J Adv Res. 2020 Oct 21;29:207-221. doi: 10.1016/j.jare.2020.10.003. eCollection 2021 Mar. J Adv Res. 2020. PMID: 33842017 Free PMC article. Review.
-
CRISPR/Cas: a Nobel Prize award-winning precise genome editing technology for gene therapy and crop improvement.J Zhejiang Univ Sci B. 2021 Apr 15;22(4):253-284. doi: 10.1631/jzus.B2100009. J Zhejiang Univ Sci B. 2021. PMID: 33835761 Free PMC article. Review.
-
CRISPR/Cas Genome Editing and Precision Plant Breeding in Agriculture.Annu Rev Plant Biol. 2019 Apr 29;70:667-697. doi: 10.1146/annurev-arplant-050718-100049. Epub 2019 Mar 5. Annu Rev Plant Biol. 2019. PMID: 30835493 Review.
-
[Application of CRISPR/Cas9 mediated gene editing in trees].Yi Chuan. 2020 Jul 20;42(7):657-668. doi: 10.16288/j.yczz.20-092. Yi Chuan. 2020. PMID: 32694105 Review. Chinese.
-
CRISPR/Cas as a Genome-Editing Technique in Fruit Tree Breeding.Int J Mol Sci. 2023 Nov 23;24(23):16656. doi: 10.3390/ijms242316656. Int J Mol Sci. 2023. PMID: 38068981 Free PMC article. Review.
Cited by
-
CRISPR/Cas9 ribonucleoprotein mediated DNA-free genome editing in larch.For Res (Fayettev). 2024 Oct 31;4:e036. doi: 10.48130/forres-0024-0033. eCollection 2024. For Res (Fayettev). 2024. PMID: 39552837 Free PMC article.
-
Identification of U6 Promoter and Establishment of Gene-Editing System in Larix kaempferi (Lamb.) Carr.Plants (Basel). 2024 Dec 26;14(1):45. doi: 10.3390/plants14010045. Plants (Basel). 2024. PMID: 39795305 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
