Demethylzeylasteral induces PD-L1 ubiquitin-proteasome degradation and promotes antitumor immunity via targeting USP22
- PMID: 39525573
- PMCID: PMC11544192
- DOI: 10.1016/j.apsb.2024.08.004
Demethylzeylasteral induces PD-L1 ubiquitin-proteasome degradation and promotes antitumor immunity via targeting USP22
Abstract
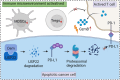
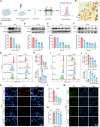
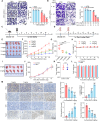
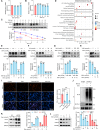
Programmed cell death ligand-1 (PD-L1) is a T cell inhibitory immune checkpoint molecule that interacts with programmed cell death-1 (PD-1) to promote immune escape of tumor cells. Compared with antibody therapies, small molecule drugs show better prospects due to their advantages such as higher bioavailability, better tissue penetration, and reduced risk of immunogenicity. Here, we found that the small molecule demethylzeylasteral (Dem) can significantly downregulate the expression of PD-L1 in colorectal cancer cells and enhance the killing effect of T cells on tumor cells. Mechanistically, Dem binds to the deubiquitinating enzyme USP22 and promotes its degradation, resulting in increased ubiquitination and degradation of PD-L1 through the proteasome pathway. In addition, Dem increased the activity of cytotoxic T cells and reduced the number of myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) in tumor-infiltrating lymphocytes (TILs), thereby activating the tumor immune microenvironment and inhibiting the growth of subcutaneous MC38 tumors in C57BL/6 mice. Moreover, we also found that the combination of Dem and CTLA4 antibodies can further improve the efficacy of antitumor therapy. Our study reveals the mechanism by which Dem promotes PD-L1 degradation and suggests that the combination of Dem and CTLA4 antibodies may improve the efficacy of immunotherapy.
Keywords: Antitumor immunity; Colorectal cancer; Demethylzeylasteral; Deubiquitination; Immune checkpoint blockade; PD-L1; Tumor-infiltrating lymphocytes; USP22.
© 2024 The Authors.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures


References
-
- Yamaguchi H., Hsu J.M., Yang W.H., Hung M.C. Mechanisms regulating PD-L1 expression in cancers and associated opportunities for novel small-molecule therapeutics. Nat Rev Clin Oncol. 2022;19:287–305. - PubMed
-
- Dong H.D., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B., et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. - PubMed
-
- Zou W.P., Chen L.P. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. - PubMed
-
- Inman B.A., Longo T.A., Ramalingam S., Harrison M.R. Atezolizumab: a PD-L1-blocking antibody for bladder cancer. Clin Cancer Res. 2017;23:1886–1890. - PubMed
-
- Robert C., Schachter J., Long G.V., Arance A., Grob J.J., Mortier L., et al. Pembrolizumab versus ipilimumab in advanced melanoma. New Engl J Med. 2015;372:2521–2532. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

