Disease-derived circulating extracellular vesicle preconditioning: A promising strategy for precision mesenchymal stem cell therapy
- PMID: 39525589
- PMCID: PMC11544168
- DOI: 10.1016/j.apsb.2024.06.027
Disease-derived circulating extracellular vesicle preconditioning: A promising strategy for precision mesenchymal stem cell therapy
Abstract
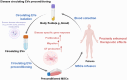
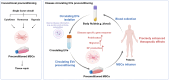
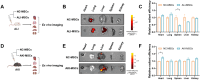
Mesenchymal stem cell (MSC)-based therapies have emerged as promising methods for regenerative medicine; however, how to precisely enhance their tissue repair effects is still a major question in the field. Circulating extracellular vesicles (EVs) from diseased states carry diverse pathological information and affect the functions of recipient cells. Based on this unique property, we report that disease-derived circulating EV (disease-EV) preconditioning is a potent strategy for precisely enhancing the tissue repair potency of MSCs in diverse disease models. Briefly, plasma EVs from lung or kidney tissue injuries were shown to contain distinctly enriched molecules and were shown to induce tissue injury-specific gene expression responses in cultured MSCs. Disease-EV preconditioning improved the performance (including proliferation, migration, and growth factor production) of MSCs through metabolic reprogramming (such as via enhanced oxidative phosphorylation and lipid metabolism) without inducing an adverse immune response. Consequently, compared with normal MSCs, disease-EV-preconditioned MSCs exhibited superior tissue repair effects (including anti-inflammatory and antiapoptotic effects) in diverse types of tissue injury (such as acute lung or kidney injury). Disease-derived EVs may serve as a type of "off-the-shelf" product due to multiple advantages, such as flexibility, stability, long-term storage, and ease of shipment and use. This study highlights the idea that disease-EV preconditioning is a robust strategy for precisely enhancing the regenerative capacity of MSC-based therapies.
Keywords: Cell death; Circulating extracellular vesicle; Inflammation; Mesenchymal stem cell; Metabolic reprogramming; Preconditioning; Regenerative medicine; Tissue injury.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no competing interests in this work.
Figures




References
LinkOut - more resources
Full Text Sources

