Target fishing and mechanistic insights of the natural anticancer drug candidate chlorogenic acid
- PMID: 39525590
- PMCID: PMC11544177
- DOI: 10.1016/j.apsb.2024.07.005
Target fishing and mechanistic insights of the natural anticancer drug candidate chlorogenic acid
Abstract
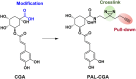
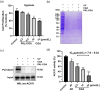
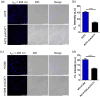
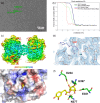
Chlorogenic acid (CGA) is a natural product that effectively inhibits tumor growth, demonstrated in many preclinical models, and phase II clinical trials for patients with glioma. However, its direct proteomic targets and anticancer molecular mechanisms remain unknown. Herein, we developed a novel bi-functional photo-affinity probe PAL/CGA and discovered mitochondrial acetyl-CoA acetyltransferase 1 (ACAT1) was one of the main target proteins of CGA by using affinity-based protein profiling (AfBPP) chemical proteomic approach. We performed in-depth studies on ACAT1/CGA interactions via multiple assays including SPR, ITC, and cryo-EM. Importantly, we demonstrated that CGA impaired cancer cell proliferation by inhibiting the phosphorylation of tetrameric ACAT1 on Y407 residue through a novel mode of action in vitro and in vivo. Our study highlights the use of AfBPP platforms in uncovering unique druggable modalities accessed by natural products. And identifying the molecular target of CGA sheds light on the future clinical application of CGA for cancer therapy.
Keywords: ACAT1; AfBPP; Chlorogenic acid; Natural product; Probe.
© 2024 The Authors.
Conflict of interest statement
Jie Zhang is a shareholder in Sichuan Jiuzhang Biological Science and Technology Co., Ltd. All other authors declare no competing interests.
Figures



References
-
- Clifford M.N., Jaganath I.B., Ludwig I.A., Crozier A. Chlorogenic acids and the acyl-quinic acids: discovery, biosynthesis, bioavailability and bioactivity. Nat Prod Rep. 2017;34:1391–1421. - PubMed
-
- Lu H.J., Tian Z.M., Cui Y.Y., Liu Z.C., Ma X.Y. Chlorogenic acid: a comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr Rev Food Sci F. 2020;19:3130–3158. - PubMed
-
- Machado F., Coimbra M.A., del Castillo M.D., Coreta-Gomes F. Mechanisms of action of coffee bioactive compounds‒a key to unveil the coffee paradox. Crit Rev Food Sci. 2023;20:1–23. - PubMed
-
- Caruso G., Torrisi S.A., Mogavero M.P., Currenti W., Castellano S., Godos J., et al. Polyphenols and neuroprotection: therapeutic implications for cognitive decline. Pharmacol Therapeut. 2022;232 - PubMed
-
- Xue H.Y., Wei M.J., Ji L.L. Chlorogenic acids: a pharmacological systematic review on their hepatoprotective effects. Phytomedicine. 2023;118 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

