Didang Tang alleviates neuronal ferroptosis after intracerebral hemorrhage by modulating the PERK/eIF2α/ATF4/CHOP/GPX4 signaling pathway
- PMID: 39525631
- PMCID: PMC11544539
- DOI: 10.3389/fphar.2024.1472813
Didang Tang alleviates neuronal ferroptosis after intracerebral hemorrhage by modulating the PERK/eIF2α/ATF4/CHOP/GPX4 signaling pathway
Abstract
Introduction: Ferroptosis is a crucial process contributing to neuronal damage following intracerebral hemorrhage (ICH). Didang Tang (DDT), a traditional therapeutic, has been used clinically to manage ICH for many years, yet the molecular mechanisms by which by DDT protects neurons from ferroptosis after ICH remain elusive.
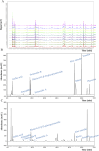
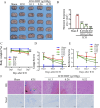
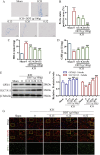
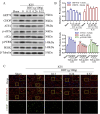
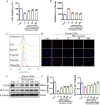
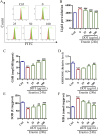
Methods: This study utilized high-performance liquid chromatography-based fingerprint analysis to characterize DDT's chemical composition. An ICH rat model and hemin and erastin-induced PC12 cell ferroptosis models were developed to investigate DDT's neuroprotective mechanisms. Histological assessments of brain tissue morphology and iron deposition were performed using hematoxylin-eosin, Nissl, and Perl's blue staining. Neurological function was evaluated using Longa and Berderson scores, while lipid peroxidation was measured using biochemical assays and flow cytometry. Protein expression levels of ferroptosis- and endoplasmic reticulum stress (ERS)-related markers were analyzed via Western blotting and immunofluorescence.
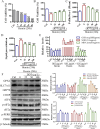
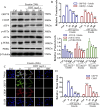
Results: Our results demonstrated that DDT reduced hematoma volume, decreased iron deposition, lowered malondialdehyde (MDA) levels, and upregulated glutathione peroxidase (GPX4) and SLC7A11 expression in affected brain regions. Furthermore, DDT downregulated GRP78 expression and inhibited the PERK/eIF2α/ATF4/CHOP/GPX4 pathway, exerting strong neuroprotective effects. The fluorescence staining results of MAP2/GPX4 and MAP2/CHOP suggested that DDT may regulate neuronal ferroptosis and ERs to exert the protective effect. In vitro experiments using hemin- and erastin-induced neuron-derived PC12 cells as neuronal ferroptosis models developed in our laboratory corroborated these in vivo findings, showing increased survival and reduced lipid peroxidation in DDT-treated cells, along with similar inhibitory effects on ferroptosis and ERS. Molecular docking suggested that DDT components, such as sennoside B, amygdalin, rhein, and emodin, interact favorably with PERK/eIF2α/ATF4/CHOP signaling pathway proteins, highlighting their potential role in DDT's anti-ferroptosis effects.
Conclusion: DDT alleviates neuronal ferroptosis after ICH by modulating the PERK/eIF2α/ATF4/CHOP/GPX4 signaling pathway. Overall, this study provides novel insights into DDT's protective mechanisms against ICH-induced neuronal injury by modulating ferroptosis and ERS pathways, underscoring its potential as an effective therapeutic strategy.
Keywords: Didang Tang; HPLC; endoplasmic reticulum stress; ferroptosis; intracerebral hemorrhage.
Copyright © 2024 Lu, Xu, Li, Tang, Zhang, Zhang, Xu, Sun and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Di-Dang-Tang suppresses ferroptosis in the hippocampal CA1 region by targeting PGK1/NRF2/GPX4 signaling pathway to exert neuroprotection in vascular dementia.Int Immunopharmacol. 2025 Mar 26;150:114233. doi: 10.1016/j.intimp.2025.114233. Epub 2025 Feb 12. Int Immunopharmacol. 2025. PMID: 39946772
-
DiDang Tang Inhibits Endoplasmic Reticulum Stress-Mediated Apoptosis Induced by Oxygen Glucose Deprivation and Intracerebral Hemorrhage Through Blockade of the GRP78-IRE1/PERK Pathways.Front Pharmacol. 2018 Dec 4;9:1423. doi: 10.3389/fphar.2018.01423. eCollection 2018. Front Pharmacol. 2018. PMID: 30564125 Free PMC article.
-
Endoplasmic Reticulum Stress Promotes Neuronal Damage in Neonatal Hypoxic-Ischemic Brain Damage by Inducing Ferroptosis.Mol Biotechnol. 2025 Feb;67(2):805-815. doi: 10.1007/s12033-024-01095-9. Epub 2024 Feb 8. Mol Biotechnol. 2025. PMID: 38329706
-
Rhein attenuates cerebral ischemia-reperfusion injury via inhibition of ferroptosis through NRF2/SLC7A11/GPX4 pathway.Exp Neurol. 2023 Nov;369:114541. doi: 10.1016/j.expneurol.2023.114541. Epub 2023 Sep 14. Exp Neurol. 2023. PMID: 37714424
-
Ferroptosis-associated pathological injury mechanisms and therapeutic strategies after intracerebral hemorrhage.Front Neurol. 2025 May 1;16:1508718. doi: 10.3389/fneur.2025.1508718. eCollection 2025. Front Neurol. 2025. PMID: 40376154 Free PMC article. Review.
Cited by
-
Traditional Chinese Medicine and Ferroptosis in Intracerebral Hemorrhage: A Potential Therapeutic Approach.Drug Des Devel Ther. 2025 Jun 4;19:4789-4808. doi: 10.2147/DDDT.S513343. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40486126 Free PMC article. Review.
-
The interplay of ferroptosis and oxidative stress in the pathogenesis of aortic dissection.Front Pharmacol. 2025 Feb 5;16:1519273. doi: 10.3389/fphar.2025.1519273. eCollection 2025. Front Pharmacol. 2025. PMID: 39974735 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

