Transcriptomics of Subcutaneous Tissue of Lipedema Identified Differentially Expressed Genes Involved in Adipogenesis, Inflammation, and Pain
- PMID: 39525887
- PMCID: PMC11548906
- DOI: 10.1097/GOX.0000000000006288
Transcriptomics of Subcutaneous Tissue of Lipedema Identified Differentially Expressed Genes Involved in Adipogenesis, Inflammation, and Pain
Abstract
Background: Lipedema is a disease typically affecting women with a symmetrical, painful fat distribution disorder, which is hypothesized to be caused by impaired adipogenesis, inflammation, and extracellular matrix remodeling, leading to fibrosis and the development of edema in lipedema subcutaneous adipose tissue. The pathogenesis and molecular processes leading to lipedema have not yet been clarified.
Methods: A whole transcriptome analysis of subcutaneous tissue of lipedema stages I (n = 12), II (n = 9), and III (n = 8) compared with hypertrophied subcutaneous tissue (n = 4) was performed. Further data about hormonal substitution and body morphology were collected. The study is registered at ClinicalTrials.gov (NCT05861583).
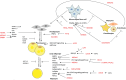
Results: We identified several differentially expressed genes involved in mechanisms leading to the development of lipedema. Some genes, such as PRKG2, MEDAG, CSF1R, BICC1, ERBB4, and ACP5, are involved in adipogenesis, regulating the development of mature adipocytes from mesenchymal stem cells. Other genes, such as MAFB, C1Q, C2, CD68, CD209, CD163, CD84, BCAT1, and TREM2, are predicted to be involved in lipid accumulation, hypertrophy, and the inflammation process. Further genes such as SHTN1, SCN7A, and SCL12A2 are predicted to be involved in the regulation and transmission of pain.
Conclusions: In summary, the pathogenesis and development of lipedema might be caused by alterations in adipogenesis, inflammation, and extracellular matrix remodeling, leading to fibrosis and the formation of edema resulting in this painful disease. These processes differ from hypertrophied adipose tissue and may therefore play a main role in the formation of lipedema.
Copyright © 2024 The Authors. Published by Wolters Kluwer Health, Inc. on behalf of The American Society of Plastic Surgeons.
Conflict of interest statement
The authors have no financial interest to declare in relation to the content of this article. The study was sponsored by the University Hospital Schleswig-Holstein, Luebeck, Germany. The patient counseling and sample collection of this study were supported by Hanse Clinic, Luebeck, Germany.
Figures


References
-
- Herpertz U. Das Lipödem [Lipedema]. Z Lymphol. 1995;19:1–11. [Article in German.] - PubMed
-
- Reich-Schupke S, Schmeller W, Brauer WJ, et al. . S1-Leitlinie Lipodem. J Dtsch Dermatol Ges. 2017;15:758–768. - PubMed
-
- Meier-Vollrath I, Schmeller W. [Lipoedema—current status, new perspectives]. J Dtsch Dermatol Ges. 2004;2:181–186. - PubMed
-
- Child AH, Gordon KD, Sharpe P, et al. . Lipedema: an inherited condition. Am J Med Genet A. 2010;152A:970–976. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
