Ultrasound-stimulated Microbubbles for Treatment of Pancreatic Cancer Cells with Radiation and Nanoparticles: In vitro Study
- PMID: 39526155
- PMCID: PMC11548062
- DOI: 10.4103/jmp.jmp_30_24
Ultrasound-stimulated Microbubbles for Treatment of Pancreatic Cancer Cells with Radiation and Nanoparticles: In vitro Study
Abstract
Purpose: This study aims to investigate the radiation enhancement effects of ultrasound-stimulated microbubbles (USMB) with X-rays and nanoparticles on pancreatic cancer cells in vitro.
Methods: Sonazoid™ microbubbles were used for USMB treatment with a commercially available ultrasound unit. The characterization of the microbubbles before and after ultrasound exposure with different mechanical parameters was evaluated microscopically. Two pancreatic cancer cell lines, MIAPaCa-2 and PANC-1, were treated with different concentrations of microbubbles in combination with 150 kVp X-rays and hydrogen peroxide-modified titanium dioxide nanoparticles. Cell viability was evaluated using a water-soluble tetrazolium dye and a colony formation assay. In addition, intracellular reactive oxygen species (ROS) induced by the combined treatment were assessed.
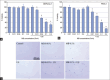
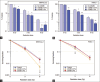
Results: The number of burst microbubbles increased with ultrasound's higher mechanical index and the exposure time. A significant radiation enhancement effect with a significant increase in ROS levels was observed in MIAPaCa-2 cells treated with USMB and 6 Gy X-rays, whereas it was not significant in PANC-1 cells treated with the same. When a higher concentration of USMB was applied with X-rays, no radiation enhancement effects were observed in either cell line. Moreover, there was no radiation enhancement effect by USMB between cells treated with and without nanoparticles.
Conclusions: The results indicate that USMB treatment can additively enhance the therapeutic efficacy of radiation therapy on pancreatic cancer cells, while the synergistic enhancement effects are likely to be cell type and microbubble concentration dependent. In addition, USMB did not improve the efficacy of nanoparticle-induced radiosensitization in the current setting.
Keywords: Microbubbles; nanoparticles; radiation therapy; radiosensitizer; ultrasound.
Copyright: © 2024 Journal of Medical Physics.
Conflict of interest statement
There are no conflicts of interest.
Figures






References
-
- Calliada F, Campani R, Bottinelli O, Bozzini A, Sommaruga MG. Ultrasound contrast agents: Basic principles. Eur J Radiol. 1998;27(Suppl 2):S157–60. - PubMed
-
- Chong WK, Papadopoulou V, Dayton PA. Imaging with ultrasound contrast agents: Current status and future. Abdom Radiol (NY) 2018;43:762–72. - PubMed
-
- Stride E, Segers T, Lajoinie G, Cherkaoui S, Bettinger T, Versluis M, et al. Microbubble agents: New directions. Ultrasound Med Biol. 2020;46:1326–43. - PubMed
LinkOut - more resources
Full Text Sources
