Early palliative care and overall survival in patients with metastatic upper gastrointestinal cancers (EPIC): a multicentre, open-label, randomised controlled phase 3 trial
- PMID: 39526177
- PMCID: PMC11544378
- DOI: 10.1016/j.eclinm.2024.102470
Early palliative care and overall survival in patients with metastatic upper gastrointestinal cancers (EPIC): a multicentre, open-label, randomised controlled phase 3 trial
Abstract
Background: Early palliative care (EPC) leads to an improvement in quality of life and an unexpected survival benefit compared with oncological care for patients with metastatic lung cancer. The Early Palliative Integrated Care (EPIC) is aimed at examining whether EPC can improve overall survival in patients with metastatic upper gastrointestinal cancer.
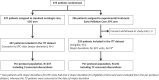
Methods: We performed a multicentre, open-label, randomised phase-3 trial. Eligible patients were ≥18 years, had metastatic upper gastrointestinal cancer and a performance status of 0-2. Patients from 19 French centres were randomly assigned between 10/10/2016 and 17/12/2021 to receive EPC plus oncological care or standard oncological care (SOC) alone. EPC was provided by palliative care physicians and included five EPC visits scheduled every month, starting within 3 weeks after randomisation. The primary endpoint was overall survival, analysed by intention-to-treat. This study was registered at ClinicalTrials.gov (NCT02853474).
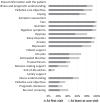
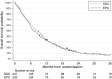
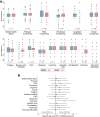
Findings: 470 patients were randomised: 233 and 237 patients in the EPC and SOC groups, respectively. In the EPC group, 216/233 patients (92.7%) underwent ≥1EPC visit, with 159 EPC visits per protocol (68.2%). The median follow-up duration was 46 months. We did not observe any overall survival difference between the EPC (median = 7.0 months [95% confidence interval, 6.1-8.8]) and SOC groups (8.6 months [6.8-9.8]) (stratified hazard ratio = 1.04 [0.86-1.26], p = 0.68). No significant heterogeneity was found in primary tumour locations, performance status groups, sex, age groups, and inclusion periods.
Interpretation: Our findings suggested that receiving EPC did not improve the benefit of oncological care with regard to overall survival in patients with metastatic upper gastrointestinal cancer.
Funding: Programme Hospitalier de Recherche Clinique, Ligue Contre le Cancer, Conseil Régional du Nord-Pas-de-Calais.
Keywords: Advanced cancer; Early palliative care; Patient-centered care; Randomised trial; Survival; Upper gastrointestinal cancer.
© 2024 The Authors.
Conflict of interest statement
Antoine Adenis: Grants or contracts from ARCUS, AstraZeneca, Bayer Pharma, BEIGENE, BMS, Roche; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from BMS and Novartis. Support for attending meetings and/or travel from AstraZeneca, Bayer Pharma, BMS, MSD, Pierre Fabre, Servier. Participation on a Data Safety Monitoring Board or Advisory Board from Astellas, Bayer Pharma, BMS, MSD. Meher Ben Abdelghani: Support for attending meetings and/or travel from Servier, Merck, Amgen, Roche. Participation on a Data Safety Monitoring Board or Advisory Board from Incyte, Deciphera, Bayer, Pierre Fabre, Servier, BMS. Vincent Bourgeois: Grants or contracts from Servier. Support for attending meetings and/or travel from Ipsen, Astra Zeneca, Sanofi. Participation on a Data Safety Monitoring Board or Advisory Board from AstraZeneca, Pierre Fabre, Servier. Anthony Turpin: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Servier, BMS, MSD, Viatris, AstraZeneca, Daiichi. Marie-Pierre Galais: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Servier, BMS. Support for attending meetings and/or travel from Servier, Bayer, AAA, Merck. Emmanuelle Samalin: Grants or contracts from Merck, Bayer, Servier. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Pierre Fabre Oncology; MSD, Amgen, BMS. Support for attending meetings and/or travel from Pierre Fabre Oncology; Servier, MSD. Participation on a Data Safety Monitoring Board or Advisory Board from Astellas, Servier, MSD. Sahir Javed: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca. Support for attending meetings and/or travel from Lilly, Pfizer. Delphine Cornuault-Foubert: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from BMS. Support for attending meetings and/or travel from Vifor, Amgen, Leopharma, Mundipharma. Christine Belletier: Support for attending meetings and/or travel from Mundipharma, Servier and Viatris. Nicolas Penel: Grants or contracts from Bayer.
Figures

References
-
- WHO Definition of palliative care. https://www.who.int/news-room/fact-sheets/detail/palliative-care/en/
-
- Ferrell B.R., Temel J.S., Temin S., et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35:96–112. - PubMed
-
- Jordan K., Aapro M., Kaasa S., et al. European Society for Medical Oncology (ESMO) position paper on supportive and palliative care. Ann Oncol. 2018;29:36–43. - PubMed
-
- Temel J.S., Greer J.A., Muzikansky A., et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. - PubMed
-
- Ferrell B.R., Twaddle M.L., Melnick A., Meier D.E. National consensus project clinical practice guidelines for quality palliative care guidelines, 4th edition. J Palliat Med. 2018;21:1684–1689. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

