Associations of Circulating Platelet Endothelial Cell Adhesion Molecule-1 Levels With Progression of Cerebral Small-Vessel Disease, Cognitive Decline, and Incident Dementia
- PMID: 39526361
- PMCID: PMC11681413
- DOI: 10.1161/JAHA.124.035133
Associations of Circulating Platelet Endothelial Cell Adhesion Molecule-1 Levels With Progression of Cerebral Small-Vessel Disease, Cognitive Decline, and Incident Dementia
Abstract
Background: The association between platelet endothelial cell adhesion molecule-1 (PECAM-1) with cerebral small-vessel disease and cognition in dementia-free subjects remains uninvestigated.
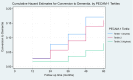
Methods and results: A prospective cohort of dementia-free subjects was recruited from memory clinics and followed up for 5 years. Annual neurocognitive assessments and twice-yearly brain magnetic resonance imaging scans were performed. Associations of baseline plasma PECAM-1 levels with cerebral small-vessel disease, cognitive decline (Montreal Cognitive Assessment scores and executive function Z scores), and incident dementia were evaluated. Of 213 subjects (aged 70.2±7.7 years, 51.2% men), median PECAM-1 levels were 0.790 (interquartile range, 0.645-0.955 ng/mL). Compared with the highest tertile, subjects within the lowest PECAM-1 tertile had greater cross-sectional white matter hyperintensity volume (β=4.84 [95% CI, 0.67-9.01]; P=0.023), age-related white matter change scores (β=1.39 [95% CI, 0.12-2.67]; P=0.033), and cerebral microbleeds (Adjusted risk ratio, 2.59 [95% CI, 1.19-5.62]; P=0.016). Of the 204 participants with follow-up data (median, 60.0 [interquartile range, 60.0-60.0] months), 24 (11.8%) developed incident dementia. Compared with the highest tertile, subjects within the lower tertiles of PECAM-1 had a higher risk of incident dementia (first tertile: adjusted hazard ratio [AHR], 4.52 [95% CI, 1.35-15.13]; P=0.024; second tertile: AHR, 3.28 [95% CI, 1.02-10.60]; P=0.047). The lowest PECAM-1 tertile was associated with greater progression of white matter hyperintensity volume (β=4.15 [95% CI, 0.06-8.24]; P=0.047), cerebral microbleeds (incident relative risk [IRR], 2.21 [95% CI, 1.05-4.65]; P=0.036), and decline in executive function (β=-0.45 [95% CI, -0.76 to -0.14]; P=0.004), and Montreal Cognitive Assessment (β=-1.32 [95% CI, -2.30 to -0.35]; P=0.008) scores.
Conclusions: In dementia-free subjects, lower circulating PECAM-1 levels are associated with greater cerebral small-vessel disease progression and cognitive decline, thus warranting future study as a potential therapeutic target.
Keywords: PECAM‐1; cerebral small‐vessel disease; cognition.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

