Modulation of SNARE-dependent exocytosis in astrocytes improves neuropathology in Huntington's disease
- PMID: 39526491
- PMCID: PMC11583919
- DOI: 10.1242/dmm.052002
Modulation of SNARE-dependent exocytosis in astrocytes improves neuropathology in Huntington's disease
Abstract
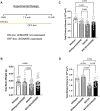
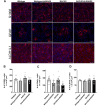
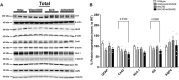
Huntington's disease (HD) is a fatal, progressive neurodegenerative disorder. Prior studies revealed an increase in extracellular glutamate levels after evoking astrocytic SNARE-dependent exocytosis from cultured primary astrocytes from mutant huntingtin (mHTT)-expressing BACHD mice compared to control astrocytes, suggesting alterations in astrocytic SNARE-dependent exocytosis in HD. We used BACHD and dominant-negative (dn)SNARE mice to decrease SNARE-dependent exocytosis from astrocytes to determine whether reducing SNARE-dependent exocytosis from astrocytes could rescue neuropathological changes in vivo. We observed significant protection against striatal atrophy and no significant rescue of cortical atrophy in BACHD/dnSNARE mice compared to BACHD mice. Amino acid transporters are important for modulating the levels of extracellular neurotransmitters. BACHD mice had no change in GLT1 expression, decreased striatal GAT1 expression and increased levels of GAT3. There was no change in GAT1 after reducing astrocytic SNARE-dependent exocytosis, and increased GAT3 expression in BACHD mice was normalized in BACHD/dnSNARE mice. Thus, modulation of astrocytic SNARE-dependent exocytosis in BACHD mice is protective against striatal atrophy and modulates GABA transporter expression.
Keywords: Astrocytes; BACHD; Huntington's disease; SNARE-dependent exocytosis; dnSNARE.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

