Quantifying neurobehavioral profiles across neurodevelopmental genetic syndromes and idiopathic neurodevelopmental disorders
- PMID: 39526825
- PMCID: PMC11965975
- DOI: 10.1111/dmcn.16112
Quantifying neurobehavioral profiles across neurodevelopmental genetic syndromes and idiopathic neurodevelopmental disorders
Abstract
Aim: To examine neurobehavioral findings in three genetic syndromes (PTEN hamartoma tumor syndrome, Malan syndrome [mutations in the NFIX gene], and SYNGAP1-related disorder), a mixed group of other neurodevelopmental genetic syndromes (NDGS), idiopathic neurodevelopmental disorder, and neurotypical control participants.
Method: Using a longitudinal case-control design, caregivers reported neurobehavioral information for 498 participants (PTEN hamartoma tumor syndrome n = 112, Malan syndrome n = 24, SYNGAP1-related disorder n = 47, other NDGS n = 72, idiopathic neurodevelopmental disorder n = 54, neurotypical siblings n = 74, and unrelated neurotypical control participants n = 115) at three timepoints (baseline, and 1-month and 4-month follow-ups) using the online-administered Neurobehavioral Evaluation Tool (NET).
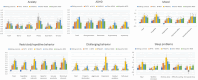
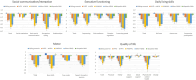
Results: NET scales had good scale and test-retest reliability. Unique patterns of neurobehavioral findings emerged, with SYNGAP1-related disorder and Malan syndrome showing generally more severe symptom and skill patterns than for other groups of patients. Patterns could be partly accounted for by estimated cognitive level, speech level, and the presence of autism spectrum disorder. However, even when accounting for these factors, group differences remained. Reliable change indices are reported.
Interpretation: Genetic syndromes associated with neurodevelopmental disorders present with unique neurobehavioral profiles that can inform selection of outcome measures in future clinical trials. The NET may be a useful screening and monitoring instrument in clinical practice, where frequent in-person clinic attendance is difficult for many patients.
© 2024 The Author(s). Developmental Medicine & Child Neurology published by John Wiley & Sons Ltd on behalf of Mac Keith Press.
Conflict of interest statement
Dr. Frazier has received funding or research support from, acted as a consultant to, received travel support from, and/or received a speaker's honorarium from the PTEN Research Foundation, SYNGAP Research Fund, Malan Syndrome Foundation, ADNP Kids Research Foundation, Quadrant Biosciences, Autism Speaks, Impel NeuroPharma, F. Hoffmann‐La Roche AG Pharmaceuticals, the Cole Family Research Fund, Simons Foundation, Ingalls Foundation, Forest Laboratories, Ecoeos, IntegraGen, KugonaLLC, Shire Development, Bristol‐Myers Squibb, Roche Pharma, MaraBio, Scioto Biosciences, National Institutes of Health, and the Brain and Behavior Research Foundation, has equity options in Quadrant Biosciences/Autism Analytica, MaraBio, and Springtide, and has an investor stake in Autism EYES LLC and iSCAN‐R. Dr. Kolevzon is on the Advisory Board for the Klingenstein Third Generation Foundation, Ovid Therapeutics, David Lynch Foundation, ADNP Kids Research Foundation, and Ritrova Therapeutics and consults to Acadia, Alkermes, Jaguar Therapeutics, GW Pharmaceuticals, Neuren Pharmaceuticals, Clinilabs Drug Development Corporation, Scioto Biosciences, and Biogen. Dr. Hardan has received funding or research support from, and acted as a consultant to, from the PTEN Research Foundation, Quadrant Biosciences, Autism Speaks, Beaming Health F. Hoffmann‐La Roche AG Pharmaceuticals, Simons Foundation, IntegraGen, and National Institutes of Health, and has equity options in Quadrant Biosciences, and has an investor stake in iSCAN‐R, and ParenteAI. TomPepper is employed by the PTEN Research Foundation who, in part, funded this research. Dr. Uljarevic has an investor stake in iSCAN‐R. Dr. Lachlan Dr. Frazier has received funding or research support from, acted as a consultantto, received travel support from, and/or received a speaker's honorarium from the PTEN Research Foundation. Dr. Harris has received funding or research support from, acted as a consultant to, and/or received travel support from the Kabuki Syndrome Foundation, KAT6 Foundation, Sotos Syndrome Support Association, Wiedemann‐Steiner Syndrome Foundation, Rubinstein‐Taybi Syndrome Childrens Foundation, and Oryzon Genomics. No other authors have conflicts of interest to disclose relevant to this manuscript. Dr. Sahin has had grant support from Biogen, Astellas, Bridgebio, and Aucta and has served on Scientific Advisory Boards for Roche, Spring Works Therapeutics, and Alkermes. He is currently on SAB for Neurogene, Jaguar Gene Therapy and Noema.
Figures
References
-
- Thomas BR, Ludwig NN, Falligant JM, Kurtz PF, Smith‐Hicks C. Severe behavior problems in SYNGAP1‐related disorder: A summary of 11 consecutive patients in a tertiary care specialty clinic. Epilepsy Behav. 2024;150:109584. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

