Human ITGAV variants are associated with immune dysregulation, brain abnormalities, and colitis
- PMID: 39526957
- PMCID: PMC11554753
- DOI: 10.1084/jem.20240546
Human ITGAV variants are associated with immune dysregulation, brain abnormalities, and colitis
Abstract
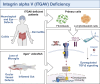
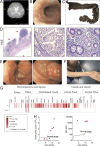
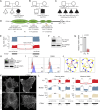
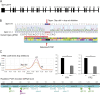
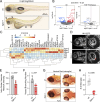
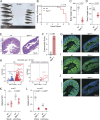
Integrin heterodimers containing an Integrin alpha V subunit are essential for development and play critical roles in cell adhesion and signaling. We identified biallelic variants in the gene coding for Integrin alpha V (ITGAV) in three independent families (two patients and four fetuses) that either caused abnormal mRNA and the loss of functional protein or caused mistargeting of the integrin. This led to eye and brain abnormalities, inflammatory bowel disease, immune dysregulation, and other developmental issues. Mechanistically, the reduction of functional Integrin αV resulted in the dysregulation of several pathways including TGF-β-dependent signaling and αVβ3-regulated immune signaling. These effects were confirmed using immunostaining, RNA sequencing, and functional studies in patient-derived cells. The genetic deletion of itgav in zebrafish recapitulated patient phenotypes including retinal and brain defects and the loss of microglia in early development as well as colitis in juvenile zebrafish with reduced SMAD3 expression and transcriptional regulation. Taken together, the ITGAV variants identified in this report caused a previously unknown human disease characterized by brain and developmental defects in the case of complete loss-of-function and atopy, neurodevelopmental defects, and colitis in cases of incomplete loss-of-function.
© 2024 Ghasempour et al.
Conflict of interest statement
Disclosures: J.E.M. Upton reported personal fees from Pharming and Pfizer, and non-financial support from Novartis outside the submitted work. J.M. Hulst reported grants from Nestle Canada, other from Abbott Canada, Mead Johnson Canada, Takeda, Baxter Canada, Fresenius Canada, and Nutricia Canada outside the submitted work. S.B. Snapper reported grants from Pfizer, Amgen, and Novartis, personal fees from Pfizer, Merck, Dualyx, Spyre Therapeutics, Biolojic Design, and Sonoma Therapeutics outside the submitted work. No other disclosures were reported.
Figures


References
MeSH terms
Substances
Grants and funding
- Canada Foundation for Innovation
- CIHR Foundation Grant
- 2015/16/T/NZ5/00168/Polish National Science Centre
- ANR-10-IAHU-01/Investissement d'Avenir
- Institut National de la Santé et de la Recherche Médicale
- Canadian Institute for Advanced Research
- Ontario Research Foundation
- Leona M. and Harry B. Helmsley Charitable Trust
- EQU202203014656/Fondation pour la Recherche Médicale
- RC2 DK122532/DK/NIDDK NIH HHS/United States
- Hospital for Sick Children
- Fondation Princesse Grace de Monaco
- RC2DK122532/NH/NIH HHS/United States
- Government of Ontario
- Lassonde Family Precision IBD Initiative
- RC2 DK118640/DK/NIDDK NIH HHS/United States
- ED562/Université Paris-Cité
- Canada Research Chair program
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

