Intratracheal Budesonide Mixed With Surfactant for Extremely Preterm Infants: The PLUSS Randomized Clinical Trial
- PMID: 39527075
- PMCID: PMC11555571
- DOI: 10.1001/jama.2024.17380
Intratracheal Budesonide Mixed With Surfactant for Extremely Preterm Infants: The PLUSS Randomized Clinical Trial
Abstract
Importance: Bronchopulmonary dysplasia (BPD) is a common adverse outcome in extremely preterm infants born at less than 28 weeks' gestation. Systemic corticosteroids are effective against BPD but may be associated with adverse outcomes. Corticosteroids given directly into the lungs may be effective and safer.
Objective: To investigate the effectiveness of early intratracheal corticosteroid administration on survival free of BPD in extremely preterm infants.
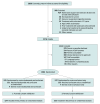
Design, setting, and participants: Double-blind randomized clinical trial conducted in 21 neonatal units in 4 countries (Australia, New Zealand, Canada, and Singapore), enrolling infants born at less than 28 weeks' gestation and less than 48 hours old who were mechanically ventilated (regardless of ventilator settings or oxygen requirements) or who were receiving noninvasive respiratory support and had a clinical decision to treat with surfactant. Recruitment occurred from January 2018 to March 2023. The last participant was discharged from the hospital in August 2023.
Interventions: Infants were randomly allocated (1:1) to receive budesonide, 0.25 mg/kg, mixed with surfactant (poractant alfa), administered via an endotracheal tube or thin catheter, or surfactant only.
Main outcomes and measures: The primary outcome was survival free of BPD at 36 weeks' postmenstrual age. There were 15 secondary outcomes, including the 2 components of the primary outcome (survival at 36 weeks and BPD among survivors), and 9 predefined safety outcomes (adverse events).
Results: The primary analysis included 1059 infants, 524 in the budesonide and surfactant group and 535 in the surfactant-only group. Overall, infants had a mean gestational age of 25.6 weeks (SD, 1.3 weeks) and a mean birth weight of 775 g (SD, 197 g); 586 (55.3%) were male. Survival free of BPD occurred in 134 infants (25.6%) in the budesonide and surfactant group and 121 infants (22.6%) in the surfactant-only group (adjusted risk difference, 2.7% [95% CI, -2.1% to 7.4%]). At 36 weeks' postmenstrual age, 83.2% of infants were alive in the budesonide and surfactant group and 80.6% in the surfactant-only group. Of these, 69.3% and 71.9% were diagnosed with BPD, respectively.
Conclusions and relevance: In extremely preterm infants receiving surfactant for respiratory distress syndrome, early intratracheal budesonide may have little to no effect on survival free of BPD.
Trial registration: anzctr.org.au Identifier: ACTRN12617000322336.
Conflict of interest statement
Figures

Comment in
- doi: 10.1001/jama.2024.19641
References
-
- Chow SSW, Creighton P, Holberton JR, Chambers GM, Lui K. Report of the Australian and New Zealand Neonatal Network 2021. ANZNN; 2023.
-
- Baker EK, Cheong J, Doyle LW. Short- and long-term outcomes after bronchopulmonary dysplasia. In: Kallapur SG, Pryhuber GS, eds. Updates on Neonatal Chronic Lung Disease. Elsevier; 2020:291-305. doi:10.1016/B978-0-323-68353-1.00020-8 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

