Efficacy and safety of intravitreal conbercept and triamcinolone acetonide for wet age-related macular degeneration in China: a meta-analysis
- PMID: 39527325
- PMCID: PMC11554939
- DOI: 10.1007/s10792-024-03346-9
Efficacy and safety of intravitreal conbercept and triamcinolone acetonide for wet age-related macular degeneration in China: a meta-analysis
Abstract
Background: Several studies have investigated the efficacy and safety of Conbercept versus Triamcinolone acetonide for the treatment of wet age-related macular degeneration (wAMD), but the results are controversial. Therefore, this meta-analysis aims to evaluate the efficacy and safety of Conbercept versus Triamcinolone acetonide for the treatment of wAMD.
Methods: A total of seven databases were searched for literature on the treatment of wAMD with Conbercept, with the search period from database inception to May 2024. Patients in the experimental group received Conbercept treatment, while patients in the control group received Triamcinolone acetonide treatment. The observed indicators included central macular thickness, incidence of adverse reactions, clinical efficacy, and best-corrected visual acuity. Relative risk (RR) and mean difference (MD) were used as effect measures.
Results: A total of 12 studies with 864 patients were included in the meta-analysis. The results showed that the experimental group had a significantly better central macular thickness (MD = - 42.68, 95% CI = - 55.04 ~ - 30.32, P < 0.001), clinical response rate (RR = 1.25, 95% CI = 1.12 ~ 1.39, P < 0.001), and best-corrected visual acuity (MD = 0.16, 95% CI = 0.12 ~ 0.20, P < 0.001) compared to the control group. In terms of adverse events, the overall incidence of adverse events was lower in the experimental group (RR = 0.26, 95% CI = 0.14 ~ 0.51, P < 0.001) compared to the control group. Specifically, the incidence of intraocular pressure elevation (RR = 0.33, 95% CI = 0.12 ~ 0.94, P = 0.04) and intraocular inflammation (RR = 0.17, 95% CI = 0.03 ~ 0.97, P = 0.05) was lower in the experimental group compared to the control group, but there was no significant difference in the incidence of subconjunctival hemorrhage (RR = 0.7, 95% CI = 0.14 ~ 3.5, P = 0.66) and corneal edema (RR = 0.2, 95% CI = 0.04 ~ 1.12, P = 0.07).
Conclusion: Conbercept demonstrated superior efficacy in treating wAMD compared to triamcinolone acetonide, with a lower incidence of adverse reactions. However, the current meta-analysis included a limited number of studies, with only two studies evaluating best-corrected visual acuity (BCVA). Further high-quality randomized controlled trials (RCTs) are warranted to validate these findings.
Keywords: Conbercept; Meta-analysis; Triamcinolone acetonide; Wet age-related macular degeneration.
© 2024. The Author(s).
Conflict of interest statement
Figures

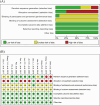
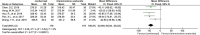


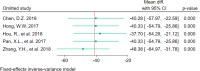
References
-
- Mukharram MB et al. (2023) Risk factors and prevalence of age-related macular degeneration according to the Russian population study in a comparative aspect with world research. In: Toчкa зpeния. Bocтoк-Зaпaд
-
- Johanna MS, Clara AC (2004) The epidemiology of age-related macular degeneration. Int Ophthalmol Clinics 44(4):17–39 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

