Glutamatergic Circuits in the Pedunculopontine Nucleus Modulate Multiple Motor Functions
- PMID: 39527367
- PMCID: PMC11607253
- DOI: 10.1007/s12264-024-01314-y
Glutamatergic Circuits in the Pedunculopontine Nucleus Modulate Multiple Motor Functions
Abstract
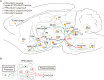
The functional role of glutamatergic (vGluT2) neurons in the pedunculopontine nucleus (PPN) in modulating motor activity remains controversial. Here, we demonstrated that the activity of vGluT2 neurons in the rostral PPN is correlated with locomotion and ipsilateral head-turning. Beyond these motor functions, we found that these rostral PPN-vGluT2 neurons remarkably respond to salient stimuli. Furthermore, we systematically traced the upstream and downstream projections of these neurons and identified two downstream projections from these neurons to the caudal pontine reticular nucleus/anterior gigantocellular reticular nucleus (PnC/GiA) and the zona incerta (ZI). Our findings indicate that the projections to the PnC/GiA inhibit movement, consistent with 'pause-and-play' behavior, whereas those to the ZI promote locomotion, and others respond to a new 'pause-switch-play' pattern. Collectively, these findings elucidate the multifaceted influence of the PPN on motor functions and provide a robust theoretical framework for understanding its physiological and potential therapeutic implications.
Keywords: Anterior gigantocellular reticular nucleus; Caudal pontine reticular nucleus; Glutamatergic neuron; Pause-and-play; Pedunculopontine nucleus; Zona incerta.
© 2024. The Author(s).
Conflict of interest statement
Conflict of interest: The authors declare no competing financial interests.
Figures




Similar articles
-
Neural correlates of multidimensional motor outputs in an excitatory parafascicular-zona incerta circuit.Biochem Biophys Res Commun. 2022 Feb 5;591:102-109. doi: 10.1016/j.bbrc.2021.12.036. Epub 2021 Dec 17. Biochem Biophys Res Commun. 2022. PMID: 35007833
-
Activation of Pedunculopontine Glutamate Neurons Is Reinforcing.J Neurosci. 2017 Jan 4;37(1):38-46. doi: 10.1523/JNEUROSCI.3082-16.2016. J Neurosci. 2017. PMID: 28053028 Free PMC article.
-
Glutamatergic and cholinergic pedunculopontine neurons innervate the thalamic parafascicular nucleus in rats: changes following experimental parkinsonism.Brain Struct Funct. 2011 Nov;216(4):319-30. doi: 10.1007/s00429-011-0317-x. Epub 2011 Apr 16. Brain Struct Funct. 2011. PMID: 21499800
-
The Cellular Diversity of the Pedunculopontine Nucleus: Relevance to Behavior in Health and Aspects of Parkinson's Disease.Neuroscientist. 2017 Aug;23(4):415-431. doi: 10.1177/1073858416682471. Epub 2016 Dec 7. Neuroscientist. 2017. PMID: 27932591 Review.
-
Implications of gamma band activity in the pedunculopontine nucleus.J Neural Transm (Vienna). 2016 Jul;123(7):655-665. doi: 10.1007/s00702-015-1485-2. Epub 2015 Nov 23. J Neural Transm (Vienna). 2016. PMID: 26597124 Free PMC article. Review.
Cited by
-
Inhibitory basal ganglia nuclei differentially innervate pedunculopontine nucleus subpopulations and evoke differential motor and valence behaviors.bioRxiv [Preprint]. 2025 Apr 19:2024.08.05.606694. doi: 10.1101/2024.08.05.606694. bioRxiv. 2025. Update in: Elife. 2025 Aug 20;13:RP102308. doi: 10.7554/eLife.102308. PMID: 39149277 Free PMC article. Updated. Preprint.
-
Inhibitory basal ganglia nuclei differentially innervate pedunculopontine nucleus subpopulations and evoke differential motor and valence behaviors.Elife. 2025 Aug 20;13:RP102308. doi: 10.7554/eLife.102308. Elife. 2025. PMID: 40833252 Free PMC article.
-
Special Issue Celebrating the 25th Anniversary of the Institute of Neuroscience, CAS.Neurosci Bull. 2024 Nov;40(11):1599-1601. doi: 10.1007/s12264-024-01318-8. Epub 2024 Nov 13. Neurosci Bull. 2024. PMID: 39538094 No abstract available.
-
Chronic Chemogenetic Activation of Astrocytes in the Murine Mesopontine Region Leads to Disturbances in Circadian Activity and Movement.Int J Mol Sci. 2025 May 16;26(10):4793. doi: 10.3390/ijms26104793. Int J Mol Sci. 2025. PMID: 40429935 Free PMC article.
References
-
- Parkinson J. An essay on the shaking palsy. J Neuropsychiatry Clin Neurosci 2002, 14: 223–236. - PubMed
-
- Shik ML, Severin FV, Orlovskiĭ GN. Control of walking and running by means of electric stimulation of the midbrain. Biofizika 1966, 11: 659–666. - PubMed
-
- Ryczko D, Dubuc R. The multifunctional mesencephalic locomotor region. Curr Pharm Des 2013, 19: 4448–4470. - PubMed
-
- Fasano A, Aquino CC, Krauss JK, Honey CR, Bloem BR. Axial disability and deep brain stimulation in patients with Parkinson disease. Nat Rev Neurol 2015, 11: 98–110. - PubMed
-
- Thevathasan W, Debu B, Aziz T, Bloem BR, Blahak C, Butson C. Pedunculopontine nucleus deep brain stimulation in Parkinson’s disease: A clinical review. Mov Disord 2018, 33: 10–20. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

