Darolutamide in Japanese patients with metastatic hormone-sensitive prostate cancer: Phase 3 ARASENS subgroup analysis
- PMID: 39527466
- PMCID: PMC11552649
- DOI: 10.1002/cam4.70029
Darolutamide in Japanese patients with metastatic hormone-sensitive prostate cancer: Phase 3 ARASENS subgroup analysis
Abstract
Background: In the global ARASENS study (NCT02799602), darolutamide plus androgen-deprivation therapy (ADT) and docetaxel significantly reduced risk of death by 32.5% versus placebo plus ADT and docetaxel (hazard ratio [HR] 0.68; 95% confidence interval [CI] 0.57-0.80; p < 0.0001), with a favorable safety profile in patients with metastatic hormone-sensitive prostate cancer (mHSPC). We investigated outcomes in Japanese participants.
Methods: Patients were randomized 1:1 to oral darolutamide 600 mg twice daily or placebo, plus ADT and docetaxel. The primary endpoint was overall survival.
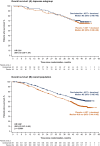
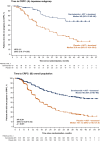
Results: The Japanese subgroup comprised 148 patients (darolutamide 63, placebo 85). In the Japanese versus overall population, more patients were aged ≥75 years (darolutamide/placebo 35%/22% vs. 16%/17%) and had body mass index <25 kg/m2 (78%/79% vs. 46%/43%), The ECOG performance status 0 (92%/88% vs. 72%/71%), de novo mHSPC (95%/97% vs. 86%/87%), and Gleason score ≥8 (94%/92% vs. 78%/79%). Median treatment duration was 43.3/15.4 months for darolutamide/placebo. The overall survival HR for darolutamide versus placebo was 0.91 (95% CI 0.50-1.64), despite 85% of patients in the placebo group receiving subsequent life-prolonging therapy. Darolutamide prolonged time to castration-resistant prostate cancer (HR 0.31; 95% CI 0.17-0.55). Treatment-emergent adverse event incidences were generally similar between groups. Adverse events known to be associated with docetaxel (e.g., neutropenia) were more frequent in the Japanese versus overall population.
Conclusion: In conclusion, efficacy outcomes showed positive trends for darolutamide plus ADT and docetaxel in Japanese patients with mHSPC, consistent with the overall population, despite higher risk factors. The combination was well tolerated, with no new safety signals in Japanese patients.
Keywords: Japanese; darolutamide; efficacy; metastatic hormone‐sensitive prostate cancer; safety.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Motohide Uemura, Hiroaki Kikukawa, Yasuhiro Hashimoto, and Hiroji Uemura have no conflict of interest; Atsushi Mizokami received fees, honoraria, and remuneration from Bayer; Masashi Kato has no conflict of interest; Hisashi Matsushima received fees and honoraria from Janssen; Takeo Kosaka, Motonobu Nakamura, and Satoshi Fukasawa have no conflict of interest; Matthew R. Smith received fees, honoraria, and remuneration from Bayer; Bertrand Tombal received fees, honoraria, and remuneration from Astellas, Bayer, Janssen, and Sanofi; Maha Hussain received fees and honoraria from Astellas and Bayer, and research funds paid to her institution from Bayer; Fred Saad received fees and honoraria from Astellas, Bayer, Janssen, and Sanofi, and research funding paid to his institution from Astellas, Bayer, Janssen, and Sanofi; Karim Fizazi received fees and honoraria from Astellas, Bayer, Janssen, and Sanofi; Cora N. Sternberg received fees and honoraria from Astellas and Bayer; E. David Crawford received fees, honoraria, and remuneration from Bayer; Haruka Kakiuchi, Masanao Akiyama, Rui Li, and Iris Kuss received remuneration from Bayer (employees); Heikki Joensuu received remuneration from Orion Pharma, is Chair of the Scientific Advisory Board of Neutron Therapeutics, and owns stock in Orion Pharma and Sartar Therapeutics; Hiroyoshi Suzuki received fees, honoraria, and remuneration from Bayer and Sanofi. The study was designed under the responsibility of Bayer and Orion Pharma, in conjunction with the protocol steering committee; the study was funded by Bayer and Orion Pharma; darolutamide was provided by Bayer; Bayer collected and analyzed the data and contributed to the interpretation of the study. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

