A novel HIV triple broadly neutralizing antibody (bNAb) combination-based passive immunization of infant rhesus macaques achieves durable protective plasma neutralization levels and mediates anti-viral effector functions
- PMID: 39527587
- PMCID: PMC11554116
- DOI: 10.1371/journal.pone.0312411
A novel HIV triple broadly neutralizing antibody (bNAb) combination-based passive immunization of infant rhesus macaques achieves durable protective plasma neutralization levels and mediates anti-viral effector functions
Abstract
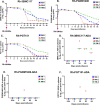
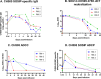
To eliminate vertical HIV transmission and achieve therapy-free viral suppression among children living with HIV, novel strategies beyond antiretroviral therapy (ART) are necessary. Our group previously identified a triple broadly neutralizing antibody (bNAb) combination comprising of 3BNC117, PGDM1400 and PGT151 that mediates robust in vitro neutralization and non-neutralizing effector functions against a cross-clade panel of simian human immunodeficiency viruses (SHIVs). In this study, we evaluated the safety, pharmacokinetics, and antiviral potency of this bNAb combination in infant rhesus macaques (RMs). We demonstrate that subcutaneous infusion of the triple bNAb regimen was well tolerated in pediatric monkeys and resulted in durable systemic and mucosal distribution. Plasma obtained from passively-immunized RMs demonstrated potent HIV-neutralizing and Fc-mediated antiviral effector functions. Finally, using the predicted serum neutralization 80% inhibitory dilution titer (PT80) biomarker threshold of >200, which was recently identified as a surrogate endpoint for evaluation of the preventative efficacy of bNAbs against mucosal viral acquisition in human clinical trials, we demonstrated that our regimen has PT80>200 against a large panel of plasma and breast milk-derived HIV strains and cross-clade SHIV variants. This data will guide the development of combination bNAbs for eliminating vertical HIV transmission and for achieving ART-free viral suppression among children living with HIV.
Copyright: © 2024 Dankwa et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
“S.R.P serves as a consultant for Merck, Moderna, Pfizer, Hoopika, Dynavax, and GSK on their CMV vaccine programs, and has led sponsored programs with Merck and Moderna on CMV vaccines. Other authors have no conflict of interest to disclose. This does not alter our adherence to PLOS ONE policies on sharing data and materials.”
Figures


Similar articles
-
Protective Efficacy of Broadly Neutralizing Antibodies with Incomplete Neutralization Activity against Simian-Human Immunodeficiency Virus in Rhesus Monkeys.J Virol. 2017 Sep 27;91(20):e01187-17. doi: 10.1128/JVI.01187-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28768869 Free PMC article.
-
Systematic Assessment of Antiviral Potency, Breadth, and Synergy of Triple Broadly Neutralizing Antibody Combinations against Simian-Human Immunodeficiency Viruses.J Virol. 2021 Jan 13;95(3):e01667-20. doi: 10.1128/JVI.01667-20. Print 2021 Jan 13. J Virol. 2021. PMID: 33177194 Free PMC article.
-
Virological Control by the CD4-Binding Site Antibody N6 in Simian-Human Immunodeficiency Virus-Infected Rhesus Monkeys.J Virol. 2017 Jul 27;91(16):e00498-17. doi: 10.1128/JVI.00498-17. Print 2017 Aug 15. J Virol. 2017. PMID: 28539448 Free PMC article.
-
The Use of Broadly Neutralizing Antibodies (bNAbs) in HIV-1 Treatment and Prevention.Viruses. 2024 Jun 4;16(6):911. doi: 10.3390/v16060911. Viruses. 2024. PMID: 38932203 Free PMC article. Review.
-
Broadly Neutralizing Antibodies for HIV-1 Prevention.Front Immunol. 2021 Jul 20;12:712122. doi: 10.3389/fimmu.2021.712122. eCollection 2021. Front Immunol. 2021. PMID: 34354713 Free PMC article. Review.
Cited by
-
Structural development of the HIV-1 apex-directed PGT145-PGDM1400 antibody lineage.Cell Rep. 2025 Jan 28;44(1):115223. doi: 10.1016/j.celrep.2024.115223. Epub 2025 Jan 17. Cell Rep. 2025. PMID: 39826122 Free PMC article.
References
-
- UNAIDS. Global HIV & AIDS statistics—Fact sheet 2023. [cited 2022]. Available from: https://www.unaids.org/en/resources/fact-sheet.
-
- Zoungrana-Yameogo WN, Fassinou LC, Ngwasiri C, Samadoulougou S, Traoré IT, Hien H, et al.. Adherence to HIV Antiretroviral Therapy Among Pregnant and Breastfeeding Women, Non-Pregnant Women, and Men in Burkina Faso: Nationwide Analysis 2019–2020. Patient Prefer Adherence. 2022;16:1037–47. Epub 2022/04/22. doi: 10.2147/PPA.S354242 ; PubMed Central PMCID: PMC9013679. - DOI - PMC - PubMed
-
- Humphrey JH, Marinda E, Mutasa K, Moulton LH, Iliff PJ, Ntozini R, et al.. Mother to child transmission of HIV among Zimbabwean women who seroconverted postnatally: prospective cohort study. Bmj. 2010;341:c6580. Epub 2010/12/24. doi: 10.1136/bmj.c6580 ; PubMed Central PMCID: PMC3007097 form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

