A modular protein language modelling approach to immunogenicity prediction
- PMID: 39527593
- PMCID: PMC11581412
- DOI: 10.1371/journal.pcbi.1012511
A modular protein language modelling approach to immunogenicity prediction
Abstract
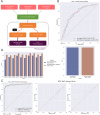
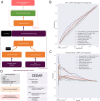
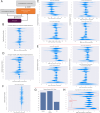
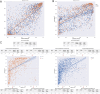
Neoantigen immunogenicity prediction is a highly challenging problem in the development of personalised medicines. Low reactivity rates in called neoantigens result in a difficult prediction scenario with limited training datasets. Here we describe ImmugenX, a modular protein language modelling approach to immunogenicity prediction for CD8+ reactive epitopes. ImmugenX comprises of a pMHC encoding module trained on three pMHC prediction tasks, an optional TCR encoding module and a set of context specific immunogenicity prediction head modules. Compared with state-of-the-art models for each task, ImmugenX's encoding module performs comparably or better on pMHC binding affinity, eluted ligand prediction and stability tasks. ImmugenX outperforms all compared models on pMHC immunogenicity prediction (Area under the receiver operating characteristic curve = 0.619, average precision: 0.514), with a 7% increase in average precision compared to the next best model. ImmugenX shows further improved performance on immunogenicity prediction with the integration of TCR context information. ImmugenX performance is further analysed for interpretability, which locates areas of weakness found across existing immunogenicity models and highlight possible biases in public datasets.
Copyright: © 2024 O’Brien et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: S.A.Q. is co-founder and chief scientific officer and own shares in Achilles Therapeutics. C.S. acknowledges grants from AstraZeneca, Boehringer-Ingelheim, Bristol Myers Squibb, Pfizer, Roche-Ventana, Invitae (previously Archer Dx Inc., a collaboration in minimal residual disease sequencing technologies), Ono Pharmaceutical and Personalis. He is chief investigator for the AZ MeRmaiD 1 and 2 clinical trials and is the steering committee chair. He is also co-chief investigator of the NHS Galleri trial funded by GRAIL and a paid member of GRAIL’s scientific advisory board (SAB). He receives consultant fees from Achilles Therapeutics (also an SAB member); Bicycle Therapeutics (also an SAB member); Genentech; Medicxi; the China Innovation Centre of Roche (CICoR), formerly Roche Innovation Centre Shanghai; Metabomed (until July 2022); Relay Therapeutics; and the Sarah Cannon Research Institute. C.S has received honoraria from Amgen, AstraZeneca, Bristol Myers Squibb, GlaxoSmithKline, Illumina, MSD, Novartis, Pfizer and Roche-Ventana; previously held stock options in Apogen Biotechnologies and GRAIL; currently has stock options in Epic Bioscience and Bicycle Therapeutics; and has stock options and is co-founder of Achilles Therapeutics. S.R.H. is the cofounder of PokeAcell and is co-inventor of licensed patents related to T cell detection. H.O’B., M.S., L.M. and F.O’F. are employees of Achilles Therapeutics. H.O’B. and M.S. are both named inventors on patents for ImmugenX.
Figures



References
-
- Reynisson B, Alvarez B, Paul S, Peters B, Nielsen M. NetMHCpan-4.1 and NetMHCIIpan-4.0: improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Research. 2020;48(W1):W449–W454. doi: 10.1093/nar/gkaa379 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

