Critical role of Babesia bovis spherical body protein 3 in ridge formation on infected red blood cells
- PMID: 39527619
- PMCID: PMC11581398
- DOI: 10.1371/journal.ppat.1012294
Critical role of Babesia bovis spherical body protein 3 in ridge formation on infected red blood cells
Abstract
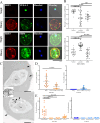
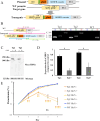
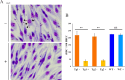
Babesia bovis, an apicomplexan intraerythrocytic protozoan parasite, causes serious economic loss to cattle industries around the world. Infection with this parasite leads to accumulation of infected red blood cells (iRBCs) in the brain microvasculature that results in severe clinical complications known as cerebral babesiosis. Throughout its growth within iRBCs, the parasite exports various proteins to the iRBCs that lead to the formation of protrusions known as "ridges" on the surface of iRBCs, which serve as sites for cytoadhesion to endothelial cells. Spherical body proteins (SBPs; proteins secreted from spherical bodies, which are organelles specific to Piroplasmida) are exported into iRBCs, and four proteins (SBP1-4) have been reported to date. In this study, we elucidated the function of SBP3 using an inducible gene knockdown (KD) system. Localization of SBP3 was assessed by immunofluorescence assay, and only partial colocalization was detected between SBP3 and SBP4 inside the iRBCs. In contrast, colocalization was observed with VESA-1, which is a major parasite ligand responsible for the cytoadhesion. Immunoelectron microscopy confirmed localization of SBP3 at the ridges. SBP3 KD was performed using the glmS system, and effective KD was confirmed by Western blotting, immunofluorescence assay, and RNA-seq analysis. The SBP3 KD parasites showed severe growth defect suggesting its importance for parasite survival in the iRBCs. VESA-1 on the surface of iRBCs was scarcely detected in SBP3 KD parasites, whereas SBP4 was still detected in the iRBCs. Moreover, abolition of ridges on the iRBCs and reduction of iRBCs cytoadhesion to the bovine brain endothelial cells were observed in SBP3 KD parasites. Immunoprecipitation followed by mass spectrometry analysis detected the host Band 3 multiprotein complex, suggesting an association of SBP3 with iRBC cytoskeleton proteins. Taken together, this study revealed the vital role of SBP3 in ridge formation and its significance in the pathogenesis of cerebral babesiosis.
Copyright: © 2024 Fathi et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Gohil S, Kats LM, Sturm A, Cooke BM. Recent insights into alteration of red blood cells by Babesia bovis: moovin’ forward. Trends Parasitol. 2010;26(12):591–9. - PubMed
-
- Aikawa M, Pongponratn E, Tegoshi T, Nakamura K, Nagatake T, Cochrane A, et al.. A study on the pathogenesis of human cerebral malaria and cerebral babesiosis. Mem Inst Oswaldo Cruz. 1992;87 Suppl 3:297–301. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

