Quorum sensing in Vibrio controls carbon metabolism to optimize growth in changing environmental conditions
- PMID: 39527643
- PMCID: PMC11581408
- DOI: 10.1371/journal.pbio.3002891
Quorum sensing in Vibrio controls carbon metabolism to optimize growth in changing environmental conditions
Abstract
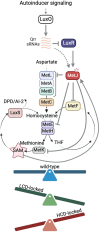
Bacteria sense population density via the cell-cell communication system called quorum sensing (QS). The evolution of QS and its maintenance or loss in mixed bacterial communities is highly relevant to understanding how cell-cell signaling impacts bacterial fitness and competition, particularly under varying environmental conditions such as nutrient availability. We uncovered a phenomenon in which Vibrio cells grown in minimal medium optimize expression of the methionine and tetrahydrofolate (THF) synthesis genes via QS. Strains that are genetically "locked" at high cell density grow slowly in minimal glucose media and suppressor mutants accumulate via inactivating mutations in metF (methylenetetrahydrofolate reductase) and luxR (the master QS transcriptional regulator). In mixed cultures, QS mutant strains initially coexist with wild-type, but as glucose is depleted, wild-type outcompetes the QS mutants. Thus, QS regulation of methionine/THF synthesis is a fitness benefit that links nutrient availability and cell density, preventing accumulation of QS-defective mutants.
Copyright: © 2024 Simpson et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Update of
-
Bacterial quorum sensing controls carbon metabolism to optimize growth in changing environmental conditions.bioRxiv [Preprint]. 2024 Jan 22:2024.01.21.576522. doi: 10.1101/2024.01.21.576522. bioRxiv. 2024. Update in: PLoS Biol. 2024 Nov 11;22(11):e3002891. doi: 10.1371/journal.pbio.3002891. PMID: 38328067 Free PMC article. Updated. Preprint.
References
-
- Tripathi S, Purchase D, Govarthanan M, Chandra R, Yadav S. Regulatory and innovative mechanisms of bacterial quorum sensing–mediated pathogenicity: a review. Environ Monit Assess. 2023;195. - PubMed
-
- Zhu J, Miller MB, Vance RE, Dziejman M, Bassler BL, Mekalanos JJ. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae The production of virulence factors including cholera toxin and the toxin-coregulated pilus in the [Internet]. 2001. Available from: www.pnas.orgcgidoi10.1073pnas.052694299.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

