Targeted engineering of camelina and pennycress seeds for ultrahigh accumulation of acetyl-TAG
- PMID: 39527733
- PMCID: PMC11588082
- DOI: 10.1073/pnas.2412542121
Targeted engineering of camelina and pennycress seeds for ultrahigh accumulation of acetyl-TAG
Abstract
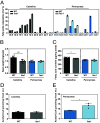
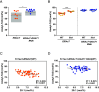
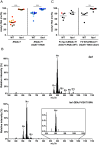
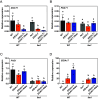
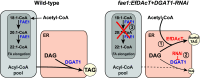
Acetyl-TAG (3-acetyl-1,2-diacylglycerol), unique triacylglycerols (TAG) possessing an acetate group at the sn-3 position, exhibit valuable properties, such as reduced viscosity and freezing points. Previous attempts to engineer acetyl-TAG production in oilseed crops did not achieve the high levels found in naturally producing Euonymus seeds. Here, we demonstrate the successful generation of camelina and pennycress transgenic lines accumulating nearly pure acetyl-TAG at 93 mol% and 98 mol%, respectively. These ultrahigh acetyl-TAG synthesizing lines were created using gene-edited FATTY ACID ELONGASE1 (FAE1) mutant lines as an improved genetic background to increase levels of acetyl-CoA available for acetyl-TAG synthesis mediated by the expression of EfDAcT, a high-activity diacylglycerol acetyltransferase isolated from Euonymus fortunei. Combining EfDAcT expression with suppression of the competing TAG-synthesizing enzyme DGAT1 further enhanced acetyl-TAG accumulation. These ultrahigh levels of acetyl-TAG exceed those in earlier engineered oilseeds and are equivalent or greater than those in Euonymus seeds. Imaging of lipid localization in transgenic seeds revealed that the low amounts of residual TAG were mostly confined to the embryonic axis. Similar spatial distributions of specific TAG and acetyl-TAG molecular species, as well as their probable diacylglycerol (DAG) precursors, provide additional evidence that acetyl-TAG and TAG are both synthesized from the same tissue-specific DAG pools. Remarkably, this ultrahigh production of acetyl-TAG in transgenic seeds exhibited minimal negative effects on seed properties, highlighting the potential for production of designer oils required for economical biofuel industries.
Keywords: biofuels; oil seeds; synthetic biology; triacylglycerols.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Kleiman R., Miller R. W., Earle F. R., Wolff I. A., (S)-1,2-diacyl-3-acetins: Optically active triglycerides from Euonymus verrucosus seed oil. Lipids 2, 473–478 (1967). - PubMed
-
- Sidorov R. A., Zhukov A. V., Pchelkin V. P., Vereshchagin A. G., Tsydendambaev V. D., Content and fatty acid composition of neutral acylglycerols in Euonymus fruits. J. Am. Oil Chem. Soc. 91, 805–814 (2014). - PubMed
-
- Liu J., et al. , Metabolic engineering of oilseed crops top produce high levels of novel acetyl glyceride oils with reduced viscosity, freezing point and calorific value. Plant Biotechnol. J. 13, 858–865 (2015). - PubMed
-
- Bansal S., Durrett T. P., Camelina sativa: An ideal platform for the metabolic engineering and field production of industrial lipids. Biochimie 120, 9–16 (2016). - PubMed
MeSH terms
Substances
Grants and funding
- DBI-1726527/NSF | Major Research Instrumentation
- DBI-1427621/NSF | Major Research Instrumentation
- P20 GM103418/GM/NIGMS NIH HHS/United States
- Student Fellowship/MOE | Saudi Arabian Cultural Mission (SACM)
- DE-SC0023142/DOE | Office of Science (SC)
- 2019-69012-29851/USDA | National Institute of Food and Agriculture (NIFA)
- 2020-67013-30897/USDA | National Institute of Food and Agriculture (NIFA)
- P20GM103418/NIH | K-IDeA Networks of Biomedical Research Excellence
- 1829365/NSF | BIO | Division of Integrative Organismal Systems (IOS)
- Hatch/Multi-State project 1013013/USDA | National Institute of Food and Agriculture (NIFA)
- 2018-67009-27374/USDA | National Institute of Food and Agriculture (NIFA)
LinkOut - more resources
Full Text Sources

