First-Line Durvalumab plus Tremelimumab Treatment for Unresectable Hepatocellular Carcinoma in Real-World Clinical Practice
- PMID: 39527933
- PMCID: PMC12324697
- DOI: 10.1159/000542517
First-Line Durvalumab plus Tremelimumab Treatment for Unresectable Hepatocellular Carcinoma in Real-World Clinical Practice
Abstract
Introduction: Durvalumab plus tremelimumab combination therapy (STRIDE regimen) is a new first-line option for unresectable hepatocellular carcinoma (uHCC), but little real-world data are available to determine which patients are most likely to respond.
Methods: This study retrospectively evaluated patients with uHCC who were treated with the STRIDE regimen as the 1st line at our hospital. The primary endpoint of the study was the objective response rate (ORR). We focused on identifying factors associated with cases that had a favorable response.
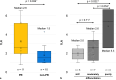
Results: Twenty-one patients were included. In best response, there were 11 partial response cases, with an ORR of 52.4%. Median progression-free survival was 6.8 months, and overall survival did not reach the median time. A high tumor-to-liver ratio of the maximum value of the standardized uptake value (TLR) on baseline fluorodeoxyglucose positron emission tomography (FDG-PET) was associated with response, while TLRs were significantly higher in poorly differentiated uHCC.
Conclusion: The STRIDE regimen may be beneficial for systemic therapy-naive uHCC patients. High TLR on baseline FDG-PET could be a potentially useful biomarker for response.
Keywords: Durvalumab; Hepatocellular carcinoma; Immune checkpoint inhibitors; Positron emission tomography; Tremelimumab.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Tomokazu Kawaoka received an honorarium from AstraZeneca K.K. and Chugai Pharmaceutical Co., Ltd.
Figures


References
-
- Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1(8):EVIDoa2100070. - PubMed
-
- Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–905. - PubMed
-
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. - PubMed
-
- Classification of Tumours Editorial Board . WHO classification of tumours of the digestive system. 5th ed. World Health Organization; 2019.
-
- World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

