Cyclosporine metabolism and pharmacokinetics following intravenous and oral administration in the dog
- PMID: 3952805
- PMCID: PMC3000222
- DOI: 10.1097/00007890-198603000-00021
Cyclosporine metabolism and pharmacokinetics following intravenous and oral administration in the dog
Abstract
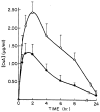
The influence of assay method on single dose cyclosporine (CsA) pharmacokinetics was studied in nine dogs receiving either i.v. or oral CsA. Samples were drawn from hepatic, portal, and systemic veins at various times after the dose and CsA levels were determined by radioimmunoassay (RIA) and high-performance liquid chromatography (HPLC). Blood concentration-time data were analyzed by nonlinear least-squares regression, using two-compartment models. RIA/HPLC ratios for all samples were greater than one, and did not change significantly over time. The mean RIA/HPLC ratios for samples drawn from all three veins were higher after oral than i.v. doses of the drug (P less than 0.05). Area under the concentration-time curve (AUC) was higher and systemic clearance (CIs) lower than calculated on the basis of RIA results, regardless of the route of administration. AUC calculated for CsA metabolites (RIA-HPLC) was highest in the portal vein after an oral dose of CsA. Bioavailability was 20.4% and 27.0% when estimated using HPLC and RIA data, respectively. The mean CsA metabolite index (CMI), when calculated for hepatic, portal, or systemic vein, was greater when the drug was administered orally. The mean hepatic extraction ratio (HER) of the parent drug and for CsA metabolites was approximately 23% in i.v. and p.o. studies. These results suggest that the gastrointestinal tract may play a role in the metabolism of CsA when the drug is administered orally. In addition, if CsA metabolites not measured by HPLC have either toxic or immunosuppressive properties, the RIA assay may be more useful for monitoring patients.
Figures

References
-
- Elliott JF, Lin Y, Mizel SB, Bleackley RC, Harnish DG, Paetkan V. Induction of Interleukin 2 messenger RNA inhibited by cyclosporin A. Science. 1984;226:1439. - PubMed
-
- Britton S, Palacios R. Cyclosporin A: Usefulness, risks and mechanism of action. Immunol Rev. 1982;65:5. - PubMed
-
- Kahan BD, Ried M, Newburger J. Pharmacokinetics of cyclosporine in human renal transplantation. Transplant Proc. 1983;15:446.
-
- Kekown PA, Stiller CR, Laupacis AL, et al. The effects and side effects of cyclosporine: relationship to drug pharmacokinetics. Transplant Proc. 1983;14:659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
