Ketone ester-enriched diet ameliorates motor and dopamine release deficits in MitoPark mice
- PMID: 39528410
- PMCID: PMC11612846
- DOI: 10.1111/ejn.16601
Ketone ester-enriched diet ameliorates motor and dopamine release deficits in MitoPark mice
Abstract
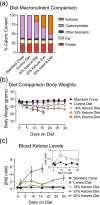
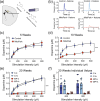
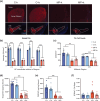
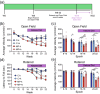
Parkinson's disease (PD) is a progressive, neurodegenerative disease characterized by motor dysfunction and dopamine deficits. The MitoPark (MP) mouse model of PD recapitulates several facets of Parkinson's disease, including gradual development of motor deficits, which enables the study of potential therapeutic interventions. One therapeutic strategy involves decreasing the mitochondrial metabolic load by inducing ketosis and providing an alternative energy source for neurons, leading to decreased neuronal oxidative stress. Thus, we hypothesized that administration of a ketone ester-enriched diet (KEED) would improve motor and dopamine release deficits in MP mice. Motor function (rotarod and open field tests), dopamine release (fast-scan cyclic voltammetry), tissue dopamine levels (gas chromatography-mass spectrometry) and dopamine neurons and axons (immunofluorescence) were assessed in MP, and control mice fed either the standard or a KEED. When started on the ketone diet before motor dysfunction onset, MP mice had improved motor function relative to standard diet (SD) MP mice. While the KEED did not preserve dopamine neurons or striatal dopamine axons, dopamine release in ketone diet MP mice was greater than SD MP mice but less than control mice. In a follow-up experiment, we began the ketone diet after motor dysfunction onset and observed a modest preservation of motor function in ketone diet MP mice relative to SD MP mice. The improvement in motor dysfunction indicates that a KEED or ketone supplement may have a beneficial effect on delaying motor deficit progression in Parkinson's disease.
Keywords: Parkinson's disease; dopamine neurons; ketosis; neuroprotection; striatum.
© 2024 The Author(s). European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
RLV and MTK received royalties from patents owned by The National Institutes of Health, Oxford University, and TdeltaS Ltd., a University of Oxford company, established to commercialize the ketone ester. All other authors declare no financial interests or potential conflicts of interest.
Figures

Similar articles
-
Manganese exposure exacerbates progressive motor deficits and neurodegeneration in the MitoPark mouse model of Parkinson's disease: Relevance to gene and environment interactions in metal neurotoxicity.Neurotoxicology. 2018 Jan;64:240-255. doi: 10.1016/j.neuro.2017.06.002. Epub 2017 Jun 20. Neurotoxicology. 2018. PMID: 28595911 Free PMC article.
-
Dopaminergic Neurons Exhibit an Age-Dependent Decline in Electrophysiological Parameters in the MitoPark Mouse Model of Parkinson's Disease.J Neurosci. 2016 Apr 6;36(14):4026-37. doi: 10.1523/JNEUROSCI.1395-15.2016. J Neurosci. 2016. PMID: 27053209 Free PMC article.
-
(+)-Borneol Protects Dopaminergic Neuronal Loss in Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-Induced Parkinson's Disease Mice: A Study of Dopamine Level using In Vivo Brain Microdialysis.ACS Chem Neurosci. 2024 Jun 5;15(11):2308-2321. doi: 10.1021/acschemneuro.4c00139. Epub 2024 May 15. ACS Chem Neurosci. 2024. PMID: 38747405
-
Voluntary exercise delays progressive deterioration of markers of metabolism and behavior in a mouse model of Parkinson's disease.Brain Res. 2019 Oct 1;1720:146301. doi: 10.1016/j.brainres.2019.146301. Epub 2019 Jun 18. Brain Res. 2019. PMID: 31226324 Free PMC article. Review.
-
RGS Proteins as Critical Regulators of Motor Function and Their Implications in Parkinson's Disease.Mol Pharmacol. 2020 Dec;98(6):730-738. doi: 10.1124/mol.119.118836. Epub 2020 Feb 3. Mol Pharmacol. 2020. PMID: 32015009 Free PMC article. Review.
References
-
- Abdelhady, R. , Saber, S. , Ahmed Abdel‐Reheim, M. , Mohammad S. Alamri, M. , Alfaifi, J. , IE Adam, M. , A. Saleh, L. , I. Farag, A. , A. Elmorsy, E. , S. El‐Wakeel, H. , & S. Doghish, A. (2023). Unveiling the therapeutic potential of exogenous beta‐hydroxybutyrate for chronic colitis in rats: Novel insights on autophagy, apoptosis, and pyroptosis. Frontiers in Pharmacology, 14, 1239025. 10.3389/fphar.2023.1239025 - DOI - PMC - PubMed
-
- Ay, M. , Luo, J. , Langley, M. , Jin, H. , Anantharam, V. , Kanthasamy, A. , & Kanthasamy, A. G. (2017). Molecular mechanisms underlying protective effects of quercetin against mitochondrial dysfunction and progressive dopaminergic neurodegeneration in cell culture and MitoPark transgenic mouse models of Parkinson's disease. Journal of Neurochemistry, 141, 766–782. 10.1111/jnc.14033 - DOI - PMC - PubMed
-
- Bender, A. , Krishnan, K. J. , Morris, C. M. , Taylor, G. A. , Reeve, A. K. , Perry, R. H. , Jaros, E. , Hersheson, J. S. , Betts, J. , Klopstock, T. , Taylor, R. W. , & Turnbull, D. M. (2006). High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nature Genetics, 38, 515–517. 10.1038/ng1769 - DOI - PubMed
-
- Boison, D. , Curtis, W. , Kemper, M. , Miller, A. , Pawlosky, R. , King, M. T. , & Veech, R. L. (2016). Ketogenic diet and metabolic therapies expanded roles in health and disease mitigation of damage from reactive oxygen species and ionizing radiation by ketone body esters. Oxford University Press.
-
- Carrarini, C. , Russo, M. , Dono, F. , Di Pietro, M. , Rispoli, M. G. , Di Stefano, V. , Ferri, L. , Barbone, F. , Vitale, M. , Thomas, A. , Sensi, S. L. , Onofrj, M. , & Bonanni, L. (2019). A stage‐based approach to therapy in Parkinson's disease. Biomolecules, 9, 388. 10.3390/biom9080388 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

