In-utero exposure to tenofovir-containing pre-exposure prophylaxis and bone mineral content in HIV-unexposed infants in South Africa
- PMID: 39528419
- PMCID: PMC11554427
- DOI: 10.1002/jia2.26379
In-utero exposure to tenofovir-containing pre-exposure prophylaxis and bone mineral content in HIV-unexposed infants in South Africa
Abstract
Introduction: Tenofovir disoproxil fumarate (TDF) is a common drug of choice for pre-exposure prophylaxis (PrEP) or as a combination HIV treatment for pregnant women. In-utero exposure to TDF was found to be associated with lower bone mineral content (BMC) in HIV-exposed uninfected neonates. Data for infants born to women taking TDF-PrEP are lacking. The CAP016 randomized control trial was conducted in South Africa between September 2017 and August 2021 and pregnant women either initiated TDF/FTC PrEP in pregnancy (Immediate PrEP arm-IP) or at cessation of breastfeeding (Deferred PrEP arm-DP). In a secondary data analysis, we evaluated BMC in HIV-unexposed infants in the CAP016 trial in the first 18 months of life in association with maternal TDF-PrEP use during pregnancy.
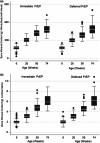
Methods: Infants born to women randomized to the IP arm or DP arm in the CAP016 clinical trial had BMC measurements of the whole body with head (WBH) and lumbar spine (LS) by dual energy X-ray absorptiometry (DXA) at 6, 26, 50 and 74 weeks.
Results: Of 481 infants born to women enrolled in the CAP016 clinical trial, 335 (69.6%) infants had a minimum of one DXA scan of the WBH and LS between 6 and 74 weeks of age (168 IP and 167 DP). Women in the IP arm received TDF-FTC PreP for a median of 19 weeks between initiation in pregnancy and delivery. Using a mixed linear regression model and adjusted for gestational age, sex and ever-breastfed, the mean difference (95% CI) for BMC of the WBH between IP and DP arms were -0.74 (-8.69 to 7.20), -1.26 (-10.75 to 8.23), -9.17 (-20.02 to 1.69) and 5.02 (-6.74 to 16.78) g at 6, 26, 50 and 74 weeks (p = 0.283). Mean differences in BMC of the LS were 0.07 (-0.10 to 0.23), 0.02 (-0.18 to 0.22), -0.14 (-0.36 to 0.09) and 0.14 (-0.11 to 0.38) g at 6, 26, 50 and 74 weeks, respectively (p = 0.329).
Conclusions: In a randomized controlled trial, there were no differences in BMC of the WBH and LS between infants exposed to in-utero TDF-FTC PrEP and unexposed infants in the first 18 months of life.
Keywords: bone mineral content; breastfeeding; infants; in‐utero exposure; pre‐exposure prophylaxis; tenofovir disoproxil fumarate.
© 2024 The Author(s). Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of International AIDS Society.
Conflict of interest statement
JFR and RC declare that they are employees of Gilead Sciences Inc and received stock options as part of their compensation packages. All other authors declare no competing interests.
KR, KLN and DM conceptualized the study, provided oversight of the formal analysis of study data and wrote the first draft of the manuscript.
CL conducted the formal analysis of the study data.
JFR, RC and GG provided financial support and resources for the project and assisted with the design of the research.
ZG and ACD played a key role in the conduct of the research and data collection.
All authors reviewed, commented on and approved this manuscript before submission for publication.
Figures
References
-
- IN DANGER: UNAIDS Global AIDS Update 2022. Geneva: Joint United Nations Programme on HIV/AIDS; 2022.
-
- WHO . Technical brief: Preventing HIV during pregnancy and breastfeeding in the context of pre‐exposure prophylaxis (PrEP). Geneva: World Health Organization; 2017.
-
- Fafin C, Pugliese P, Durant J, Mondain V, Rahelinirina V, De Salvador F, et al. Increased time exposure to tenofovir is associated with a greater decrease in estimated glomerular filtration rate in HIV patients with kidney function of less than 60 ml/min/1.73 m. Nephron Clin Pract. 2012;120:c205–c214. - PubMed
-
- Laprise C, Baril J‐G, Dufresne S, Trottier H. Association between tenofovir exposure and reduced kidney function in a cohort of HIV‐positive patients: results from 10 years of follow‐up. Clin Infect Dis. 2013;56:567–575. - PubMed
-
- Baranek B, Wang S, Cheung AM, Mishra S, Tan DH. The effect of tenofovir disoproxil fumarate on bone mineral density: a systematic review and meta‐analysis. Antivir Ther. 2020;25(1):21–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

