ASC specks as a single-molecule fluid biomarker of inflammation in neurodegenerative diseases
- PMID: 39528447
- PMCID: PMC11555386
- DOI: 10.1038/s41467-024-53547-0
ASC specks as a single-molecule fluid biomarker of inflammation in neurodegenerative diseases
Abstract
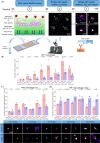
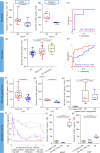
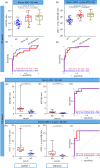
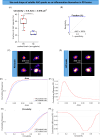
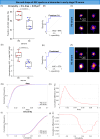
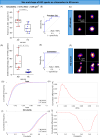
Immunotherapeutic strategies for Alzheimer's and Parkinson's disease would be facilitated by better measures of inflammation. Here we established an ultra-sensitive single-molecule pull-down immunoassay combined with direct stochastic optical reconstruction microscopy (dSTORM) to measure the number, size and shape of individual extracellular inflammasome ASC specks. We assayed human post-mortem brain, serum and cerebrospinal fluid of patients with Parkinson's and Alzheimer's as well as healthy elderly. The number of ASC specks increased and showed altered morphology in the blood of early-stage Parkinson's and Alzheimer's patients compared to controls, mimicking those found in the brain and cerebrospinal fluid. In serum samples we also measured the number of Aβ, p-tau and α-syn aggregates and formed a composite biomarker of (ASC + p-tau)/Aβ and (ASC + α-syn)/Aβ ratios that distinguished age-matched healthy controls from patients with early-stage Alzheimer's with AUC of 92% and early-stage Parkinson's with AUC of 97%. Our findings confirm ASC specks as a fluid candidate biomarker of inflammation for neurodegenerative diseases with blood being the main focus for further development as convenient sample for diagnostics and clinical trials.
© 2024. The Author(s).
Conflict of interest statement
Figures

References
-
- Aarsland, D. et al. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Prim.7, 47 (2021). - PubMed
-
- Porsteinsson, A. P., Isaacson, R. S., Knox, S., Sabbagh, M. N. & Rubino, I. Diagnosis of early Alzheimer’s disease: clinical practice in 2021. J. PreventionAlzheimer’s Dis.8, 371–386 (2021). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

