p14ARF forms meso-scale assemblies upon phase separation with NPM1
- PMID: 39528457
- PMCID: PMC11555371
- DOI: 10.1038/s41467-024-53904-z
p14ARF forms meso-scale assemblies upon phase separation with NPM1
Abstract
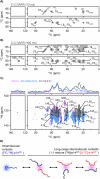
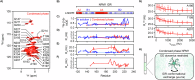
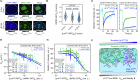
NPM1 is an abundant nucleolar chaperone that, in addition to facilitating ribosome biogenesis, contributes to nucleolar stress responses and tumor suppression through its regulation of the p14 Alternative Reading Frame tumor suppressor protein (p14ARF). Oncogenic stress induces p14ARF to inhibit MDM2, stabilize p53 and arrest the cell cycle. Under non-stress conditions, NPM1 stabilizes p14ARF in nucleoli, preventing its degradation and blocking p53 activation. However, the mechanisms underlying the regulation of p14ARF by NPM1 are unclear because the structural features of the p14ARF-NPM1 complex were elusive. Here we show that p14ARF assembles into a gel-like meso-scale network upon phase separation with NPM1. This assembly is mediated by intermolecular contacts formed by hydrophobic residues in an α-helix and β-strands within a partially folded N-terminal portion of p14ARF. These hydrophobic interactions promote phase separation with NPM1, enhance p14ARF nucleolar partitioning, restrict NPM1 diffusion within condensates and nucleoli, and reduce cellular proliferation. Our structural analysis provides insights into the multifaceted chaperone function of NPM1 in nucleoli by mechanistically linking the nucleolar localization of p14ARF to its partial folding and meso-scale assembly upon phase separation with NPM1.
© 2024. The Author(s).
Conflict of interest statement
Figures


Update of
-
p14ARF forms meso-scale assemblies upon phase separation with NPM1.Res Sq [Preprint]. 2023 Dec 7:rs.3.rs-3592059. doi: 10.21203/rs.3.rs-3592059/v1. Res Sq. 2023. Update in: Nat Commun. 2024 Nov 11;15(1):9531. doi: 10.1038/s41467-024-53904-z. PMID: 38106181 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

