A cyclic peptide toolkit reveals mechanistic principles of peptidylarginine deiminase IV regulation
- PMID: 39528459
- PMCID: PMC11555231
- DOI: 10.1038/s41467-024-53554-1
A cyclic peptide toolkit reveals mechanistic principles of peptidylarginine deiminase IV regulation
Abstract
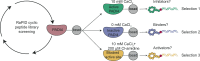
Peptidylarginine deiminase IV (PADI4, PAD4) deregulation promotes the development of autoimmunity, cancer, atherosclerosis and age-related tissue fibrosis. PADI4 additionally mediates immune responses and cellular reprogramming, although the full extent of its physiological roles is unexplored. Despite detailed molecular knowledge of PADI4 activation in vitro, we lack understanding of its regulation within cells, largely due to a lack of appropriate systems and tools. Here, we develop and apply a set of potent and selective PADI4 modulators. Using the mRNA-display-based RaPID system, we screen >1012 cyclic peptides for high-affinity, conformation-selective binders. We report PADI4_3, a cell-active inhibitor specific for the active conformation of PADI4; PADI4_7, an inert binder, which we functionalise for the isolation and study of cellular PADI4; and PADI4_11, a cell-active PADI4 activator. Structural studies with PADI4_11 reveal an allosteric binding mode that may reflect the mechanism that promotes cellular PADI4 activation. This work contributes to our understanding of PADI4 regulation and provides a toolkit for the study and modulation of PADI4 across (patho)physiological contexts.
© 2024. The Author(s).
Conflict of interest statement
Figures



References
-
- Vossenaar, E. R., Zendman, A. J. W., van Venrooij, W. J. & Pruijn, G. J. M. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. BioEssays25, 1106–1118 (2003). - PubMed
-
- Cuthbert, G. L. et al. Histone deimination antagonizes arginine methylation. Cell118, 545–553 (2004). - PubMed
-
- Wang, Y. et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science306, 279–283 (2004). - PubMed
-
- Hagiwara, T., Nakashima, K., Hirano, H., Senshu, T. & Yamada, M. Deimination of arginine residues in nucleophosmin/B23 and histones in HL-60 granulocytes. Biochem. Biophys. Res. Commun.290, 979–983 (2002). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- SRA/PRJNA1167468
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

