Deciphering influence of donor age on adipose-derived stem cells: in vitro paracrine function and angiogenic potential
- PMID: 39528480
- PMCID: PMC11555058
- DOI: 10.1038/s41598-024-73875-x
Deciphering influence of donor age on adipose-derived stem cells: in vitro paracrine function and angiogenic potential
Abstract
Background: As fat grafting is commonly used as a filler, Adipose-derived stem/stromal cells (ASC) have been reported to be key player in retention rate. Paracrine and differentiation potential of those cells confer them strong pro-angiogenic capacities. However, a full characterization of the influence of aging on ASC has not been reported yet. Here we've investigated the effect of age on paracrine function, stemness and angiogenic potential of ASC.
Methods: ASC were extracted from young and old adult donors. We assessed stromal vascular fraction cell populations repartition, ASC stemness potential, capability to differentiate into mesenchymal lineages as well as their secretome. Angiogenic potential was assessed using a sprouting assay, an indirect co-culture of ASC and dermal microvascular endothelial cells (EC). Total vascular sprout length was measured, and co-culture soluble factors were quantified. Pro-angiogenic factors alone or in combination as well as ASC-conditioned medium (CM) were added to EC to assess sprouting induction.
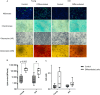
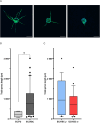
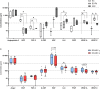
Results: Decrease of endothelial cells yield and percentage is observed in cells extracted from adipose tissue of older patients, whereas ASC percentage increased with age. Clonogenic potential of ASC is stable with age. ASC can differentiate into adipocytes, chondrocytes and osteoblasts, and aging does not alter this potential. Among the 25 analytes quantified, high levels of pro-angiogenic factors were found, but none is significantly modulated with age. ASC induce a significantly longer vascular sprouts compared to fibroblasts, and no difference was found between young and old ASC donors on that parameter. Higher concentrations of FGF-2, G-CSF, HGF and IL-8, and lower concentrations of VEGF-C were quantified in EC/ASC co-cultures compared to EC/fibroblasts co-cultures. EC/ASC from young donors secrete higher levels of VEGF-A compared to old ones. Neither soluble factor nor CM without cells are able to induce organized sprouts, highlighting the requirement of cell communication for sprouting. CM produced by ASC supporting development of long vascular sprouts promote sprouting in co-cultures that establish shorter sprouts.
Conclusion: Our results show cells from young and old donors exhibit no difference in all assessed parameters, suggesting all patients could be included in clinical applications. We emphasized the leading role of ASC in angiogenesis, without impairment with age, where secretome is a key but not sufficient actor.
Keywords: Adipose-derived stem/stromal cells; Aging; Angiogenesis; Fat graft; Paracrine.
© 2024. The Author(s).
Conflict of interest statement
Figures



References
-
- Trotzier, C., Sequeira, I., Auxenfans, C. & Mojallal, A. A. Fat graft retention: Adipose tissue, adipose-derived stem cells, and aging. Plast. Reconstr .Surg. 151(3), 420e-31e (2023). 10.1097/PRS.0000000000009918 - PubMed
-
- Charles-de-Sa, L. et al. Antiaging treatment of the facial skin by fat graft and adipose-derived stem cells. Plast. Reconstr. Surg. 135 (4), 999–1009. 10.1097/PRS.0000000000001123 (2015). - PubMed
-
- Coleman, S. R. & Katzel, E. B. Fat grafting for facial filling and regeneration. Clin. Plast. Surg. 42(3), 289–300 vii (2015). 10.1016/j.cps.2015.04.001 - PubMed
-
- Del Papa, N. et al. Autologous fat grafting in the treatment of fibrotic perioral changes in patients with systemic sclerosis. Cell. Transpl. 24 (1), 63–72. 10.3727/096368914X674062 (2015). - PubMed
-
- van Dongen, J. A. et al. The effects of facial lipografting on skin quality: A systematic review. Plast. Reconstr. Surg. 144 (5), 784e–97e. 10.1097/PRS.0000000000006147 (2019). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

