Expression of key cytokines in dog macrophages infected by Leishmania tarentolae opening new avenues for the protection against Leishmania infantum
- PMID: 39528528
- PMCID: PMC11554803
- DOI: 10.1038/s41598-024-78451-x
Expression of key cytokines in dog macrophages infected by Leishmania tarentolae opening new avenues for the protection against Leishmania infantum
Abstract
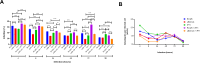
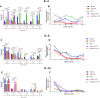
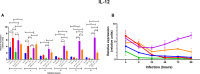
The detection of Leishmania tarentolae in sympatric areas where Leishmania infantum is endemic raised questions regarding the protective effect exerted in dogs by L. tarentolae when in coinfection. This study aimed monitoring the in vitro gene expression of pro- (IFN- γ; TNF-α; IL-12) and anti-inflammatory (IL-4; IL-6; IL-10) cytokines in primary canine macrophages infected by L. tarentolae and L. infantum in single and in co-infections. Macrophages differentiated from dog blood mononuclear cells were infected with the L. tarentolae field-isolated (RI-325) and laboratory (LEM-124) strains, with L. infantum laboratory strain (IPT1), or both. Infection and the number of amastigotes per infected cell were evaluated microscopically by counting a total of 200 cells between 4 and 96 h. Cytokine gene expression was analyzed by real-time PCR from infected macrophages mRNA. Single infections presented higher expression of the cytokines IL-4 and IL-6, and lower of IL-12. Co-infections induced a lower gene expression of IL-4 and IL-6, and a higher gene expression of IL-12, correlating with the low amastigote burden despite the slight increase of infected cells. Data highlight the potential protective effect of L. tarentolae against L. infantum in co-infection by the reduced anti-inflammatory and increased pro-inflammatory cytokines gene expression, opening new perspectives for a canine vaccine development exploiting the non-pathogenic L. tarentolae.
© 2024. The Author(s).
Conflict of interest statement
Figures
Similar articles
-
Intracellular persistence of Leishmania tarentolae in primary canine macrophage cells.Acta Trop. 2023 Jul;243:106935. doi: 10.1016/j.actatropica.2023.106935. Epub 2023 Apr 29. Acta Trop. 2023. PMID: 37127215
-
Detection of Leishmania tarentolae in lizards, sand flies and dogs in southern Italy, where Leishmania infantum is endemic: hindrances and opportunities.Parasit Vectors. 2021 Sep 8;14(1):461. doi: 10.1186/s13071-021-04973-2. Parasit Vectors. 2021. PMID: 34493323 Free PMC article.
-
Long-lasting protection against canine visceral leishmaniasis using the LiESAp-MDP vaccine in endemic areas of France: double-blind randomised efficacy field trial.Vaccine. 2007 May 22;25(21):4223-34. doi: 10.1016/j.vaccine.2007.02.083. Epub 2007 Mar 15. Vaccine. 2007. PMID: 17395339 Clinical Trial.
-
Cutaneous immune mechanisms in canine leishmaniosis due to Leishmania infantum.Vet Immunol Immunopathol. 2015 Feb 15;163(3-4):94-102. doi: 10.1016/j.vetimm.2014.11.011. Epub 2014 Nov 20. Vet Immunol Immunopathol. 2015. PMID: 25555497 Review.
-
Leishmania tarentolae: A new frontier in the epidemiology and control of the leishmaniases.Transbound Emerg Dis. 2022 Sep;69(5):e1326-e1337. doi: 10.1111/tbed.14660. Epub 2022 Aug 3. Transbound Emerg Dis. 2022. PMID: 35839512 Free PMC article. Review.
Cited by
-
Genetic Regulation of Immune Response in Dogs.Genes (Basel). 2025 Jun 29;16(7):764. doi: 10.3390/genes16070764. Genes (Basel). 2025. PMID: 40725420 Free PMC article. Review.
References
-
- Breton, M., Zhao, C., Ouellette, M., Tremblay, M. J. & Papadopoulou, B. A recombinant non-pathogenic Leishmania vaccine expressing human immunodeficiency virus 1 (HIV-1) gag elicits cell-mediated immunity in mice and decreases HIV-1 replication in human tonsillar tissue following exposure to HIV-1 infection. J. Gen. Virol.88, 217–225. 10.1099/vir.0.81995-0 (2007). - PubMed
-
- Taylor, V. M. et al. Leishmania tarentolae: utility as an in vitro model for screening of antileishmanial agents. Exp. Parasitol.126, 471–475. 10.1016/j.exppara.2010.05.016 (2010). - PubMed

