Efficacy and safety of sacituzumab govitecan Trop-2-targeted antibody-drug conjugate in solid tumors and UGT1A1*28 polymorphism: a systematic review and meta-analysis
- PMID: 39528547
- PMCID: PMC11554802
- DOI: 10.1038/s44276-024-00106-1
Efficacy and safety of sacituzumab govitecan Trop-2-targeted antibody-drug conjugate in solid tumors and UGT1A1*28 polymorphism: a systematic review and meta-analysis
Abstract
Background: Sacituzumab govitecan (SG) is a promising Trop-2-targeted antibody-drug conjugate (ADC) approved for the treatment of metastatic triple-negative breast cancer (TNBC). Early phase clinical trials have demonstrated good clinical activity and safety profile of SG in various tumor types, albeit with differing response rates and durations. The aim of this systematic review and meta-analysis was to evaluate the clinical efficacy and toxicity of SG and the influence of UGT1A1*28 genotype in clinical trials involving solid tumors.
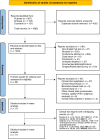
Methods: A systematic review of the literature from publicly available databases was performed on February 15, 2024 whereby studies published till 15 February 2024 were retrieved according to PRISMA guidelines [PROSPERO #CRD42022359943]. Data extracted included tumor type, sample size, demographic information, SG dose, UGT1A1*28 status, toxicity events, duration of follow-up, response, and survival outcomes. Risks of bias analysis was refereed using the Joanna Briggs Institute quality assessment tool for the cohort and RCT studies using 11 and 13 parameters, respectively. Statistical analysis was performed using the DerSimonian and Laird inverse variance methods. Heterogeneity was assessed using the I2 statistic and Χ2 tests. P value < 0.05 was considered as statistical significance.
Results: Eleven eligible clinical trials comprised of 1578 patients harboring various tumor types including TNBC, lung, genitourinary and gastrointestinal malignancies were included in the systematic review and meta-analysis. Pooled incidences of severe adverse events were minimal at <10%, with the exception of grade 3-4 neutropenia at 37.4%. The median PFS and OS across all studies were 4.9 (95%CI: 4.0-5.8) months and 9.6 (95%CI: 7.6-11.6) months, respectively. Objective response rate across all studies evaluated was 17.1% (95%CI: 12.0-22.1).
Conclusion: Our systematic review and meta-analysis confirmed that SG confers good clinical activity in certain solid tumor types and was tolerable with minimal adverse events. The potential utility of UGT1A1*28 genotyping in predicting clinical response and outcomes could not be determined due to the limited number of studies with available UGT1A1 genotype data.
© 2024. The Author(s).
Conflict of interest statement
Figures










Similar articles
-
Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial.Ann Oncol. 2021 Jun;32(6):746-756. doi: 10.1016/j.annonc.2021.03.005. Epub 2021 Mar 16. Ann Oncol. 2021. PMID: 33741442 Clinical Trial.
-
The Clinical Outcomes and Safety of Sacituzumab Govitecan in Heavily Pretreated Metastatic Triple-Negative and HR+/HER2- Breast Cancer: A Multicenter Observational Study from Turkey.Cancers (Basel). 2025 May 7;17(9):1592. doi: 10.3390/cancers17091592. Cancers (Basel). 2025. PMID: 40361516 Free PMC article.
-
Preliminary results from ASCENT-J02: a phase 1/2 study of sacituzumab govitecan in Japanese patients with advanced solid tumors.Int J Clin Oncol. 2024 Nov;29(11):1684-1695. doi: 10.1007/s10147-024-02589-x. Epub 2024 Sep 20. Int J Clin Oncol. 2024. PMID: 39302614 Free PMC article. Clinical Trial.
-
[Translated article] Influence of the UGT1A1 gene polymorphism on treatment with sacituzumab govitecan. Narrative review.Farm Hosp. 2025 May 13:S1130-6343(25)00063-7. doi: 10.1016/j.farma.2025.02.012. Online ahead of print. Farm Hosp. 2025. PMID: 40368667 Review. English, Spanish.
-
Influence of the UGT1A1 gene polymorphism on treatment with sacituzumab govitecan. Narrative review.Farm Hosp. 2025 Mar 25:S1130-6343(25)00016-9. doi: 10.1016/j.farma.2025.02.009. Online ahead of print. Farm Hosp. 2025. PMID: 40140308 Review. English, Spanish.
Cited by
-
Navigating the Complexity of Resistance in Lung Cancer Therapy: Mechanisms, Organoid Models, and Strategies for Overcoming Treatment Failure.Cancers (Basel). 2024 Nov 28;16(23):3996. doi: 10.3390/cancers16233996. Cancers (Basel). 2024. PMID: 39682183 Free PMC article. Review.
-
Efficacy and safety of Sacituzumab govitecan in solid tumors: a systematic review and meta-analysis.Front Oncol. 2025 Jun 20;15:1624386. doi: 10.3389/fonc.2025.1624386. eCollection 2025. Front Oncol. 2025. PMID: 40626021 Free PMC article.
-
Delphi consensus on the management of adverse events in patients with metastatic triple-negative breast cancer treated with sacituzumab govitecan.Oncologist. 2025 May 8;30(5):oyaf088. doi: 10.1093/oncolo/oyaf088. Oncologist. 2025. PMID: 40366333 Free PMC article.
-
Energy Decomposition-Driven Design of Trop2-Targeting Peptide.Cell Biochem Biophys. 2025 Aug 8. doi: 10.1007/s12013-025-01866-4. Online ahead of print. Cell Biochem Biophys. 2025. PMID: 40779128 No abstract available.
-
Targeting Refractory Triple-Negative Breast Cancer with Sacituzumab Govitecan: A New Era in Precision Medicine.Cells. 2024 Dec 22;13(24):2126. doi: 10.3390/cells13242126. Cells. 2024. PMID: 39768216 Free PMC article. Review.
References
-
- Sharkey RM, McBride WJ, Cardillo TM, Govindan SV, Wang Y, Rossi EA, et al. Enhanced delivery of SN-38 to human tumor xenografts with an anti-Trop-2-SN-38 antibody conjugate (Sacituzumab Govitecan). Clin Cancer Res. 2015;21:5131–8. 10.1158/1078-0432.CCR-15-0670 - PubMed
-
- Faltas B, Goldenberg DM, Ocean AJ, Govindan SV, Wilhelm F, Sharkey RM, et al. Sacituzumab govitecan, a novel antibody-drug conjugate, in patients with metastatic platinum-resistant urothelial carcinoma. Clin Genitourin Cancer. 2016;14:e75–79. 10.1016/j.clgc.2015.10.002 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
