The healing process of diabetic ulcers correlates with changes in the cutaneous microbiota
- PMID: 39528566
- PMCID: PMC11554885
- DOI: 10.1038/s41598-024-77987-2
The healing process of diabetic ulcers correlates with changes in the cutaneous microbiota
Abstract
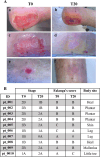
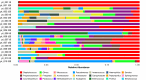
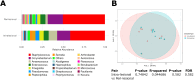
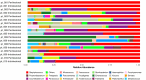
Skin microbiota plays an essential role in the development and function of the cutaneous immune system, in the maintenance of the skin barrier through the release of antimicrobial peptides, and in the metabolism of some natural products. With the aim of characterizing changes in the cutaneous microbiota specifically associated with wound healing in the diabetic condition, we performed a 16 S rRNA gene Next Generation Sequencing of skin swabs taken within the ulcer bed of ten diabetic patients before (t0) and after 20 days of therapy (t20) with a fluorescein-based galenic treatment. Considering the twenty most representative genera, we found at t20 an increase of Corynebacterium, Peptostreptococcus, and Streptococcus, and a decrease of Enterococcus, Finegoldia, and Peptoniphilus genera. However, differences were not significant due to the high variability among samples and the small patient cohort. S. aureus was the most abundant species at t0 and was reduced by therapy in four patients. Comparing the microbiome in the ulcer bed and in the perilesional tissue of the same patient at t0, no major differences were observed. Taken together, our data indicate that in the absence of antibiotic-based therapy the healing process of diabetic ulcers is accompanied by changes in the microbiome composition.
Keywords: Diabetic ulcers; Fluorescein; Microbiome; Wound healing.
© 2024. The Author(s).
Conflict of interest statement
Figures

Similar articles
-
One step closer to understanding the role of bacteria in diabetic foot ulcers: characterising the microbiome of ulcers.BMC Microbiol. 2016 Mar 22;16:54. doi: 10.1186/s12866-016-0665-z. BMC Microbiol. 2016. PMID: 27005417 Free PMC article.
-
Microbial Community Distribution and Core Microbiome in Successive Wound Grades of Individuals with Diabetic Foot Ulcers.Appl Environ Microbiol. 2020 Mar 2;86(6):e02608-19. doi: 10.1128/AEM.02608-19. Print 2020 Mar 2. Appl Environ Microbiol. 2020. PMID: 31924616 Free PMC article.
-
Quantitation and composition of cutaneous microbiota in diabetic and nondiabetic men.J Infect Dis. 2013 Apr;207(7):1105-14. doi: 10.1093/infdis/jit005. Epub 2013 Jan 8. J Infect Dis. 2013. PMID: 23300163 Free PMC article.
-
The Gut-Skin Microbiota Axis and Its Role in Diabetic Wound Healing-A Review Based on Current Literature.Int J Mol Sci. 2022 Feb 21;23(4):2375. doi: 10.3390/ijms23042375. Int J Mol Sci. 2022. PMID: 35216488 Free PMC article. Review.
-
Treatment of diabetic foot ulcers.J Cardiovasc Surg (Torino). 2009 Jun;50(3):275-91. J Cardiovasc Surg (Torino). 2009. PMID: 19543189 Review.
Cited by
-
Next-Generation Wound Care: A Scoping Review on Probiotic, Prebiotic, Synbiotic, and Postbiotic Cutaneous Formulations.Pharmaceuticals (Basel). 2025 May 9;18(5):704. doi: 10.3390/ph18050704. Pharmaceuticals (Basel). 2025. PMID: 40430523 Free PMC article. Review.
References
-
- Jeffcoate, W. et al. Causes, prevention, and management of diabetes-related foot ulcers. Lancet Diabetes Endocrinol.10.1016/s2213-8587(24)00110-4 (2024). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

