Glutamylation imbalance impairs the molecular architecture of the photoreceptor cilium
- PMID: 39528655
- PMCID: PMC11649768
- DOI: 10.1038/s44318-024-00284-1
Glutamylation imbalance impairs the molecular architecture of the photoreceptor cilium
Abstract
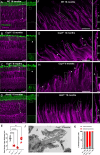
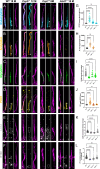
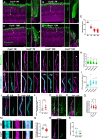
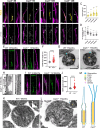
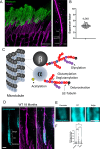
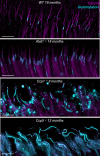
Microtubules, composed of conserved α/β-tubulin dimers, undergo complex post-translational modifications (PTMs) that fine-tune their properties and interactions with other proteins. Cilia exhibit several tubulin PTMs, such as polyglutamylation, polyglycylation, detyrosination, and acetylation, with functions that are not fully understood. Mutations in AGBL5, which encodes the deglutamylating enzyme CCP5, have been linked to retinitis pigmentosa, suggesting that altered polyglutamylation may cause photoreceptor cell degeneration, though the underlying mechanisms are unclear. Using super-resolution ultrastructure expansion microscopy (U-ExM) in mouse and human photoreceptor cells, we observed that most tubulin PTMs accumulate at the connecting cilium that links outer and inner photoreceptor segments. Mouse models with increased glutamylation (Ccp5-/- and Ccp1-/-) or loss of tubulin acetylation (Atat1-/-) showed that aberrant glutamylation, but not acetylation loss, disrupts outer segment architecture. This disruption includes exacerbation of the connecting cilium, loss of the bulge region, and destabilization of the distal axoneme. Additionally, we found significant impairment in tubulin glycylation, as well as reduced levels of intraflagellar transport proteins and of retinitis pigmentosa-associated protein RPGR. Our findings indicate that proper glutamylation levels are crucial for maintaining the molecular architecture of the photoreceptor cilium.
Keywords: Expansion Microscopy; Glutamylation; Photoreceptor Cell Cilium; Post-translational Modifications; Retinitis Pigmentosa.
© 2024. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures





References
-
- Aljammal R, Saravanan T, Guan T, Rhodes S, Robichaux MA, Ramamurthy V (2024) Excessive tubulin glutamylation leads to progressive cone-rod dystrophy and loss of outer segment integrity. Hum Mol Genet 33:802–817 - PubMed
-
- Astuti GDN, Arno G, Hull S, Pierrache L, Venselaar H, Carss K, Raymond FL, Collin RWJ, Faradz SMH, van den Born LI et al (2016) Mutations in AGBL5, encoding α-tubulin deglutamylase, are associated with autosomal recessive retinitis pigmentosa. Invest Ophthalmol Vis Sci 57:6180–6187 - PubMed
-
- Bachmann-Gagescu R, Neuhauss SC (2019) The photoreceptor cilium and its diseases. Curr Opin Genet Dev 56:22–33 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

