TRBC1-CAR T cell therapy in peripheral T cell lymphoma: a phase 1/2 trial
- PMID: 39528665
- PMCID: PMC11750712
- DOI: 10.1038/s41591-024-03326-7
TRBC1-CAR T cell therapy in peripheral T cell lymphoma: a phase 1/2 trial
Abstract
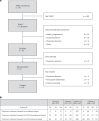
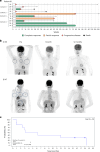
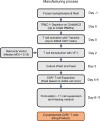
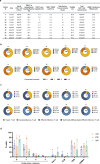
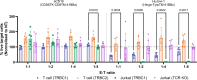
Relapsed/refractory peripheral T cell lymphomas (PTCLs) are aggressive tumors with a poor prognosis. Unlike B cell lymphomas, treatment of PTCL has not benefited from advances in immunotherapy. This is largely due to a lack of suitable target antigens that discriminate malignant from normal T cells, thus avoiding severe immunosuppression consequent to depletion of the entire T cell compartment. We recently described a targeting strategy based on the mutually exclusive expression of T cell antigen receptor beta-chain constant domain (TRBC) 1 and 2. Selective targeting of the T cell antigen receptor beta-chain expressed by the (clonal) malignancy spares normal T cells expressing the other chain. The LibraT1 study is an ongoing, multicenter, international, single-arm phase 1/2 study of TRBC1-directed autologous chimeric antigen receptor (CAR) T cells (AUTO4) in relapsed/refractory TRBC1-positive PTCL. Primary objectives were assessment of safety and tolerability of AUTO4 infusion. Key secondary endpoints included efficacy, CAR T cell expansion and persistence. Here we describe the findings from dose escalation in LibraT1 in the first ten patients, in a non-prespecified interim analysis. AUTO4 resulted in low frequency of severe immunotoxicity, with one of ten patients developing grade 3 cytokine release syndrome. Complete metabolic response was observed in four of ten evaluable patients, with remissions being durable beyond 1 year in two patients. While an absence of circulating CAR T cells was observed, CAR T cells were readily detected in lymph node biopsy samples from sites of original disease suggesting homing to tumor sites. These results support the continuing exploration of TRBC1 targeting in PTCL. ClinicalTrials.gov registration: NCT03590574 .
© 2024. The Author(s).
Conflict of interest statement
Competing interests: K.C.: consulting/advisory role: Roche, Takeda, Celgene, Atara, Gilead, KITE, Janssen, Incyte and AbbVie; speakers’ bureau: Roche, Takeda, KITE, Gilead and Incyte; conferences/travel support: Roche, Takeda, KITE, Janssen and BMS. G.I.: honoraria and travel support: Novartis, Kite/Gilead, Bristol Myers Squibb, AbbVie, Autolus, Sandoz, Miltenyi and AstraZeneca. E.T.: speaker fees: Astellas, Jazz, Kite/Gilead, Pfizer and Vertex; ad boards: Autolus, BMS/Celgene, Jazz, Janssen and Vertex. M.P.: owns stock in and has research funding and salary contribution from Autolus. D.I.: travel grants and honoraria: Kite/Gilead and Janssen. T.M.: travel grants: Amgen, Jazz, Pfizer, Bayer, Kyowa Kirin, Celgene/BMS, Kite/Gilead, Janssen and Takeda; honoraria for advisory board meetings: Kite/Gilead, Amgen, Novartis, Pfizer, Celgene/BMS, Daiichi Sankyo, Atara, Roche and Janssen; honoraria for lectures: Kite/Gilead, Takeda, Janssen, Roche, Servier, Novartis, Celgene/BMS, Pfizer and Incyte; research funding: Janssen, AstraZeneca and Novartis. T.M.: consulting/advisory role: Roche/Ventana, Haematogenix and Autolus. P.M.: Autolus: stock holder and research funding. UCL business: patent royalties. All employees of Autolus have no competing interests.
Figures



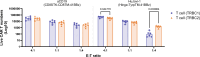
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

