TYK2 regulates tau levels, phosphorylation and aggregation in a tauopathy mouse model
- PMID: 39528671
- PMCID: PMC11614740
- DOI: 10.1038/s41593-024-01777-2
TYK2 regulates tau levels, phosphorylation and aggregation in a tauopathy mouse model
Abstract
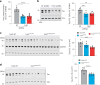
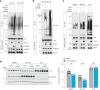
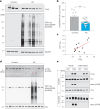
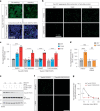
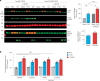
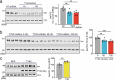
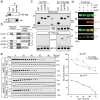
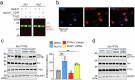
Alzheimer's disease is one of at least 26 diseases characterized by tau-positive accumulation in neurons, glia or both. However, it is still unclear what modifications cause soluble tau to transform into insoluble aggregates. We previously performed genetic screens that identified tyrosine kinase 2 (TYK2) as a candidate regulator of tau levels. Here we verified this finding and found that TYK2 phosphorylates tau at tyrosine 29 (Tyr29) leading to its stabilization and promoting its aggregation in human cells. We discovered that TYK2-mediated Tyr29 phosphorylation interferes with autophagic clearance of tau. We also show that TYK2-mediated phosphorylation of Tyr29 facilitates pathological tau accumulation in P301S tau-transgenic mice. Furthermore, knockdown of Tyk2 reduced total tau and pathogenic tau levels and rescued gliosis in a tauopathy mouse model. Collectively, these data suggest that partial inhibition of TYK2 could thus be a strategy to reduce tau levels and toxicity.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: H.Y.Z. cofounded Cajal Neuroscience, is a director of the Regeneron Pharmaceuticals board and is on the scientific advisory board of Cajal Neuroscience, Lyterian and the Column Group. D.M.H. cofounded and is on the scientific advisory board of C2N Diagnostics. D.M.H. is on the scientific advisory board of Denali, Cajal Neuroscience and Genentech, consults for Asteroid Therapeutics, is an inventor of and has a patent on antitau antibodies that was licensed by Washington University to C2N Diagnostics. B.H. owns stock in Novartis; he serves on the scientific advisory board of Dewpoint and has an option for stock. He serves on a scientific advisory board or is a consultant for AbbVie, Alexion, Ambagon, Aprinoia Therapeutics, Arvinas, Avrobio, AstraZeneca, Biogen, BMS, Cure Alz Fund, Cell Signaling Technology, Dewpoint, Latus, Novartis, Pfizer, Sanofi, Sofinnova, Vigil, Violet, Voyager and WaveBreak. His laboratory is supported by research grants from the NIH, Cure Alzheimer’s Fund, Tau Consortium and the JPB Foundation, as well as a sponsored research agreement from Abbvie. The other authors declare no competing interests.
Figures




References
-
- Williams, D. R. Tauopathies: classification and clinical update on neurodegenerative diseases associated with microtubule-associated protein tau. Intern. Med. J.36, 652–660 (2006). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

