Structural basis of the mechanism and inhibition of a human ceramide synthase
- PMID: 39528795
- PMCID: PMC11919693
- DOI: 10.1038/s41594-024-01414-3
Structural basis of the mechanism and inhibition of a human ceramide synthase
Abstract
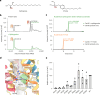
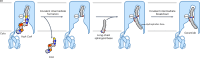
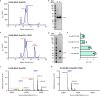
Ceramides are bioactive sphingolipids crucial for regulating cellular metabolism. Ceramides and dihydroceramides are synthesized by six ceramide synthase (CerS) enzymes, each with specificity for different acyl-CoA substrates. Ceramide with a 16-carbon acyl chain (C16 ceramide) has been implicated in obesity, insulin resistance and liver disease and the C16 ceramide-synthesizing CerS6 is regarded as an attractive drug target for obesity-associated disease. Despite their importance, the molecular mechanism underlying ceramide synthesis by CerS enzymes remains poorly understood. Here we report cryo-electron microscopy structures of human CerS6, capturing covalent intermediate and product-bound states. These structures, along with biochemical characterization, reveal that CerS catalysis proceeds through a ping-pong reaction mechanism involving a covalent acyl-enzyme intermediate. Notably, the product-bound structure was obtained upon reaction with the mycotoxin fumonisin B1, yielding insights into its inhibition of CerS. These results provide a framework for understanding CerS function, selectivity and inhibition and open routes for future drug discovery.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: C.S.T., M.T., S.T., A.P. and G.S. are employees of Boehringer Ingelheim Pharma, GmbH & Co. KG. The other authors declare no competing interests.
Figures






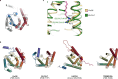





References
-
- Turpin, S. M. et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab.20, 678–686 (2014). - PubMed
-
- Apostolopoulou, M. et al. Specific hepatic sphingolipids relate to insulin resistance, oxidative stress, and inflammation in nonalcoholic steatohepatitis. Diabetes Care41, 1235–1243 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

