Open-label, controlled, phase 2 clinical trial assessing the safety, efficacy, and pharmacokinetics of INM004 in pediatric patients with Shiga toxin-producing Escherichia coli-associated hemolytic uremic syndrome
- PMID: 39528845
- PMCID: PMC12031759
- DOI: 10.1007/s00467-024-06583-3
Open-label, controlled, phase 2 clinical trial assessing the safety, efficacy, and pharmacokinetics of INM004 in pediatric patients with Shiga toxin-producing Escherichia coli-associated hemolytic uremic syndrome
Abstract
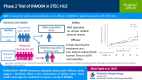
Background: Shiga toxin-producing Escherichia coli-associated hemolytic uremic syndrome (STEC-HUS) is a severe condition mainly affecting children. It is one of the leading causes of acute kidney injury in the pediatric population. There is no established therapy for this disease. INM004 is an anti-Shiga toxin composed of equine polyclonal antibodies. This study is aimed at assessing the safety, pharmacokinetics, and efficacy of INM004 in pediatric patients with STEC-HUS.
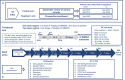
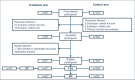
Methods: Phase 2, open-label clinical trial with an historical control arm. Patients in the treatment arm received two doses of INM004. The primary endpoints were the safety profile, pharmacokinetics, and efficacy (dialysis days) of INM004. Secondary endpoints included other kidney and extrarenal outcomes. Propensity score matching was used for efficacy comparisons between arms.
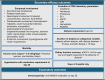
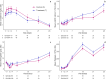
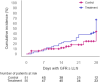
Results: Fifty-seven and 125 patients were enrolled in the treatment and control arm, respectively. After propensity score matching, 52 patients remained in each arm. INM004 was well-tolerated. Eight adverse events were considered possibly related, none of which were serious or severe. In the primary efficacy endpoint, patients of the treatment arm presented a non-statistically significant difference of two dialysis days. On secondary endpoints, non-statistically significant trends toward fewer patients needing dialysis and dialysis for more than 10 days, and shorter time to glomerular filtration rate normalization, were observed favoring the treatment arm.
Conclusions: INM004 showed an adequate safety profile. Efficacy non-statistically significant trends suggesting a beneficial effect in the amelioration of kidney injury were observed. These results encourage the conduction of a phase 3 study of INM004 in pediatric patients with STEC-HUS.
Keywords: Acute kidney injury; Clinical trial, Phase 2; Hemolytic uremic syndrome; Passive immunotherapy.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: This trial was performed following the principles of the Declaration of Helsinki and Good Clinical Practice. The protocol was approved by the local Ethics Committees of each of the participating centers and the Argentine National Food and Drug Regulatory Agency (ANMAT) after approval by the Ethics Committee accredited by the corresponding Health Authority within the jurisdiction (Disposition Number: DI-2022–7068-APN-ANMAT#MS). Conflict of interest: AF, IP, AB, LA, PC, MA, OA, MCB, MVB, PB, MLFT, LF, LG, JM, FR, CS, AS, and APS declare reimbursement for conduction of clinical trial as investigator of the study. MR, MP, VZ, LS, CM, MV, SS, MC, IR, and FG report personal fees from Inmunova S.A.
Figures
References
-
- Boyer O, Niaudet P (2022) Hemolytic-uremic syndrome in children. Pediatr Clin North Am 69:1181–1197. 10.1016/j.pcl.2022.07.006 - PubMed
-
- Fakhouri F, Zuber J, Frémeaux-Bacchi V, Loirat C (2017) Haemolytic uraemic syndrome. Lancet 390:681–696. 10.1016/S0140-6736(17)30062-4 - PubMed
-
- Bruyand M, Mariani-Kurkdjian P, Gouali M, de Valk H, Le Hello S, Bonacorsi S, Loirat C (2018) Hemolytic uremic syndrome due to Shiga toxin-producing Escherichia coli infection. Med Mal Infect 48:167–174. 10.1016/j.medmal.2017.09.012 - PubMed
-
- Ylinen E, Salmenlinna S, Halkilahti J, Jahnukainen T, Korhonen L, Virkkala T, Rimhanen-Finne R, Nuutinen M, Kataja J, Arikoski P, Linkosalo L, Bai X, Matussek A, Jalanko H, Saxén H (2020) Hemolytic uremic syndrome caused by Shiga toxin–producing Escherichia coli in children: incidence, risk factors, and clinical outcome. Pediatr Nephrol 35:1749–1759. 10.1007/s00467-020-04560-0 - PMC - PubMed
-
- Balestracci A, Meni Battaglia L, Toledo I, Martin SM, Beaudoin L (2024) Duration of prodromal phase and severity of hemolytic uremic syndrome. Pediatr Nephrol 39:213–219. 10.1007/s00467-023-06104-8 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

