Safety comparison of single-donor and pooled fecal microbiota transfer product preparation in ulcerative colitis: systematic review and meta-analysis
- PMID: 39528920
- PMCID: PMC11552227
- DOI: 10.1186/s12876-024-03487-2
Safety comparison of single-donor and pooled fecal microbiota transfer product preparation in ulcerative colitis: systematic review and meta-analysis
Abstract
Background: Multiple studies have evaluated fecal microbiota transfer (FMT) in patients with ulcerative colitis (UC) using single-donor (SDN) and multidonor (MDN) products. Systematic review and meta-analysis were performed to compare the safety of SDN and MDN products.
Methods: Systematic searches were performed in Web of Science, Scopus, PubMed, and Orbit Intelligence to identify studies that compared FMT products manufactured using SDN or MDN strategies against control treatment in patients with UC. Fifteen controlled studies were selected for meta-analysis (11 randomized controlled trials and 4 controlled cohort trials). Safety of each treatment type was assessed using the counts of adverse events and serious adverse events using fixed- and random-effects models. Significance of the indirect difference between FMT preparations was assessed using a network approach. Benefit-risk ratios were calculated by multiplicative utility model, incorporating geometric mean of risk ratios (RRs) of efficacy and safety.
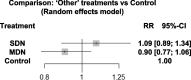
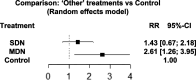
Results: Safety data was collected for a total of 587 patients (193 exposed to SDN products, 114 exposed to MDN products and 280 exposed to control treatment). The 12 studies showed similar overall safety event counts for MDN and SDN versus placebo (RRs: 0.90 and 1.09, respectively [P = 0.206 and P = 0.420, respectively]). Results indicated similar risk of safety events for MDN compared to SDN (RR: 0.83, P = 0.159). Positive benefit-risk ratios were demonstrated for MDN and SDN versus placebo (RRs: 1.70 and 1.16, respectively [P = 0.003 and P = 0.173, respectively]). MDN had a greater benefit-risk ratio compared to SDN (RR: 1.46, P = 0.072).
Conclusion: Similar safety profiles were observed for MDN and SDN strategies. Alongside previously described superior efficacy, treatment with MDN has greater benefit-risk ratio than SDN in patients with UC. Further development of MDN FMT treatment for UC should be considered.
Keywords: Benefit-risk; Fecal microbiota transfer; Meta-analysis; Pooling; Safety; Ulcerative colitis.
© 2024. The Author(s).
Conflict of interest statement
Figures
References
-
- Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. The Lancet. 2017;389(10075):1218–28. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

