Regulatory T cells require peripheral CCL2-CCR2 signaling to facilitate the resolution of medication overuse headache-related behavioral sensitization
- PMID: 39528947
- PMCID: PMC11555869
- DOI: 10.1186/s10194-024-01900-5
Regulatory T cells require peripheral CCL2-CCR2 signaling to facilitate the resolution of medication overuse headache-related behavioral sensitization
Abstract
Background: Medication overuse headache (MOH) is the most common secondary headache disorder, resulting from chronic and excessive use of medication to treat headaches, for example, sumatriptan. In a recent study, we have shown that the peripheral C-C motif ligand 2 (CCL2), C-C motif chemokine receptor 2 (CCR2) and calcitonin-gene-related peptide (CGRP) signaling pathways interact with each other and play critical roles in the development of chronic migraine-related behavioral and cellular sensitization. In the present study, we investigated whether CCL2-CCR2 and CGRP signaling pathways play a role in the development of sumatriptan overuse-induced sensitization, and whether they are involved in its resolution by the low-dose interleukin-2 (LD-IL-2) treatment.
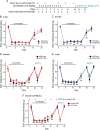
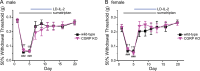
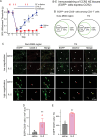
Methods: Mice received daily sumatriptan administration for 12 days. MOH-related behavioral sensitization was assessed by measuring changes of periorbital mechanical thresholds for 3 weeks. CCL2-CCR2 and CGRP signaling pathways were inhibited by targeted gene deletion or with an anti-CCL2 antibody. Ca2+-imaging was used to examine whether repetitive sumatriptan treatment enhances CGRP and pituitary adenylate cyclase-activating polypeptide (PACAP) signaling in trigeminal ganglion (TG) neurons. LD-IL-2 treatment was initiated after the establishment of sumatriptan-induced sensitization. Immunohistochemistry and flow cytometry analyses were used to examine whether CCL2-CCR2 signaling controls regulatory T (Treg) cell proliferation and/or trafficking.
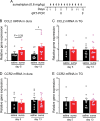
Results: CCL2, CCR2 and CGRPα global KO mice exhibited robust sumatriptan-induced behavioral sensitization comparable to wild-type controls. Antibody neutralization of peripheral CCL2 did not affect sumatriptan-induced behaviors either. Repeated sumatriptan administration did not enhance the strength of CGRP or PACAP signaling in TG neurons. Nevertheless, LD-IL-2 treatment, which facilitated the resolution of sumatriptan-induced sensitization in wild-type and CGRPα KO mice, was completely ineffective in mice with compromised CCL2-CCR2 signaling. In CCL2 KO mice, we observed normal LD-IL-2-induced Treg expansion in peripheral blood, but the increase of Treg cells in dura and TG tissues was significantly reduced in LD-IL-2-treated CCL2 KO mice relative to wild-type controls.
Conclusions: These results indicate that the endogenous CCL2-CCR2 and CGRP signaling pathways are not involved in sumatriptan-induced behavioral sensitization, suggesting that distinct molecular mechanisms underlie chronic migraine and MOH. On the other hand, peripheral CCL2-CCR2 signaling is required for LD-IL-2 to reverse chronic headache-related sensitization.
Keywords: C-C motif chemokine receptor 2 (CCR2); C-C motif ligand 2 (CCL2); Calcitonin gene-related peptide (CGRP); Facial mechanical hypersensitivity; Low-dose interleukin-2 (LD-IL-2); Medication overuse headache; Regulatory T (Treg) cell.
© 2024. The Author(s).
Conflict of interest statement
Figures




References
-
- Ashina S, Terwindt GM, Steiner TJ, Lee MJ, Porreca F, Tassorelli C, Schwedt TJ, Jensen RH, Diener HC, Lipton RB (2023) Medication overuse headache. Nat Rev Dis Primers 9(1):5 - PubMed
-
- Diener HC, Holle D, Solbach K, Gaul C (2016) Medication-overuse headache: risk factors, pathophysiology and management. Nat Reviews Neurol 12(10):575–583 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

